|
|
|
Research Article
Population viability analysis of howler monkeys (Alouatta palliata mexicana) in a highly fragmented landscape in Los Tuxtlas, MexicoSalvador Mandujano1 and Luis A. Escobedo-Morales1 1Departamento de Biodiversidad y Ecología Animal, Instituto de Ecología A. C., km 2.5 Camino Antiguo a Coatepec No. 351, Xalapa 91070, Ver., México. E-mail: [email protected]
Received: 16 January, 2008, Accepted: 16 February, 2008, Published: 3 March, 2008 Copyright: This is an open access paper. We use the Creative Commons Attribution 3.0 license http://creativecommons.org Cite this paper as: Mandujano, S. and Escobedo-Morelos, L. A. 2008. Population Viability Analysis of Howler Monkey (Alouatta palliata mexicana) in a Highly Fragmented Landscape in Los Tuxtlas, Mexico. Tropical Conservation Science 1:43-62. Available online: tropicalconservationscience.org. Introduction One of the most powerful and pervasive tools in conservation biology is population viability analysis (PVA) [1-4]. In fact, PVA has been used in conservation biology for approximately 25 years. The principal motivation behind the development of PVA is to assess the threats to a species' survival, and to intervene before population declines become inevitable [5]. In general, PVA is the study of all factors that might cause a species to go extinct [6]. Specifically, PVA could be defined as an analysis that uses data in an analytical or simulation model to calculate the risk of extinction after some specified period of time [7]. In practice, biologists use a wide variety of PVA models to interpret past population trends, to evaluate likely threats to a population, and to project future population trends [7]. Actually, there exist software programs (e.g.,Vortex, RAMAS/metapop, ALEX) that could be used to answer specific questions about population trends under different simulation scenarios. In consequence, PVA helps us not only to assess the risk of extinction but also to identify the most promising management strategies [3,6,8]. Of course, criticisms and caveats exist around PVA, defined in part by data availability and theoretical and biological understanding, and in part by social, regulatory, and political contexts [9]. The population abundance and sex-specific age structure of a primate population is the result of extrinsic natural factors (habitat, predation, competition, disease, and catastrophes) and intrinsic factors (fecundity, mortality, immigration and emigration), all of which help determine the long-term trend of population dynamics [10]. However, human-induced factors such as hunting, deforestation, and fragmentation, negatively affect population dynamics of many primate species [11]. As population size decreases, the population is more vulnerable to extinction due to demographic and environmental stochasticity, loss of heterozygosity, and disruption of social structure [12]. In this context, the PVA is a valuable analytical approach for proposing management recommendations to increase the persistence of a target primate population and species [13]. The use of PVA in primate studies has been done using different approaches [14-19]. In particular, the effect of demographic parameters on population growth has been used as an index to evaluate the importance of certain life stages for conservation [13,20]. For example, the elasticity analysis of matrix projection models examines the effects of proportional changes in demographic parameters, such as survival and fecundity rates on population growth rates [21,22]. Thus, PVA uses matrix models that require relatively few data and can be generalized for populations with a wide variety of life-history traits [20-22]. Los Tuxtlas, a region located in the Mexican state of Veracruz, is the northernmost area of lowland tropical rainforest in America [23]. These forests are inhabited by the Mexican mantled howler monkey, Alouatta palliata mexicana, and the Spider monkey, Ateles geoffroyi, both classified as critically endangered by the IUCN [24]. The reduction in distribution and abundance of primates in Los Tuxtlas is principally due to deforestation of the tropical rainforest: 75% of native habitat has been lost, and 20% has become isolated. Only 5% consists of continuous rainforest at high elevations (>800 m) [25]. As a result, the remaining population is spread across archipelagoes of forest fragments that vary in size, isolation distance, age, and quality, resulting in precarious demographic and ecological conditions [26-29]. Studies at Los Tuxtlas have shown that the presence and abundance of howlers monkeys are positively related to fragment size and quality, and negatively related to fragment isolation [18,19,25,29,30]. However, little is known about the population viability in this region. The principal objectives of the present study were development of a PVA in order to: 1) evaluate the contribution of demographic parameters to population growth, and 2) to simulate group trend and local extinction probability of Mexican mantled howler monkeys under two landscape scenarios — isolated populations and patchy population or metapopulation — in Los Tuxtlas. We discuss the PVA implications for conservation of this species. Methods Study Area
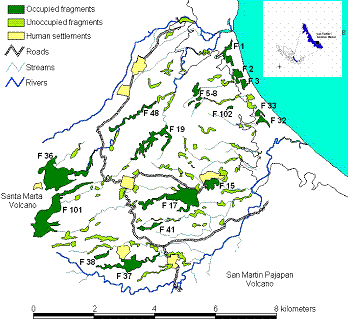
We digitized the landscape with ArcView 3.2 (ESRI®) software, using aerial photographs (1:20,000; INEGI 1999), orthophotos (INEGI 1996), and digital (INEGI 1990), and field data. We defined "fragment" as a remnant of the original forest, with a mean canopy height > 10 m, and a surface area > 0.5 ha, which appear to be the minimum parameters for patch occupation by A.p.mexicana in Los Tuxtlas [29,34]. We calculate size and isolation for all fragments using the Patch Analysis 2.2 extension. Three isolation parameters were used: nearest fragment, nearest town, and nearest continuous forest. Study species Howler monkeys (Alouatta) are known for their ability to survive in both intact and disturbed habitats of varying sizes [35]. Their broad tolerance to changes in habitat quality has been related to a high degree of folivory, a diverse and flexible diet, and the use of small home ranges [36-40]. Alouatta populations are divided into social groups that act as semi-closed reproductive units [41]. Dispersal is a reproductive strategy that is often adopted by both juveniles and adults of both sexes [42,43], possibly to avoid inbreeding and may result in maintaining the genetic variability of populations [44]. In consequence, the size and composition of the social groups vary notably in response to variation in the habitat resources and population density [41,45]. This feature has permitted howlers to survive in very small habitat fragments where other species have been unable to do so [46,47]. However, howlers are more vulnerable to hunting, diseases, and predation in a fragmented habitat [35]. Census To determine the presence and size/composition of howler monkey groups, the fragments were sampled from 07:00 to 12:00 and 16:00 to 18:00 h, during: April 2002 (10 days, 197 h), June 2002 (15 d, 260 h), October 2002 (13 d, 125 h), February 2003 (9 d, 225 h), April 2003 (7 d, 88 h), June 2003 (11d, 166 h), February 2004 (11 d, 182 h) and April 2004 (10 d, 166 h). A group was defined as a social unit having at least one adult male and one adult female. Each group encountered was followed and counted repeatedly the same day until a consensus of group size and age and sex composition was reached. All groups were followed on subsequent days to confirm their composition. Recounting groups helped to reduce the probability of counting a group more than once. The isolated nature of the forest fragments in the landscape also greatly reduced this probability. Individuals in each group were recognized by pigmentation of the hands and feet, face scars, and by patches of blond hair on hands, feet, tail, and lower back. Individuals were classified as infants (< 1 year old), juveniles (>1 year, < 3 years), adult females, and adult males (> 3 years, distinctive sexual characters). We did not distinguish sex in infants and juvenile individuals. Demographic parameters For the purposes of demographic analysis we calculated the average group composition based on the annual census from 2002 to 2004 (Appendix 1), and 24 demographic parameters for each group (Appendix 2). The complete subpopulation of animals in each occupied fragment was counted; thus sex and age composition were unbiased. The density was estimated for each occupied fragment as the number of individuals per surface area of the fragment. The ratios of female per male, and immatures per female were calculated following standardized procedure [48]. The relative reproductive success (RRS) was estimated following Jones [47]. In this case, RRS estimation was based on the average number of juveniles and infants per female group size. Thus, we calculated the RRS for groups with two, three, or more females. Fecundity and survival rates were estimated according to Akçakaya [8]. In Alouatta the births can happen during the whole year [46]. In consequence, it is possible that some infants have been born in months of the year when we didn't visit the fragments. However, these records are not relevant to fecundity rate estimation if these breedings didn't survive from t to t+1-. Thus, fecundity (Fx) is the number of newborns at time t produced by individuals in the age class x at time t-1. Specifically, Fx = Sx ´ mx , where S = survival and m = fertility. Fertility is the average number of offspring produced by an individual of age x per unit time. Thus, fertility is not the same thing as fecundity. The fecundity values incorporate two kinds of mortality over the time step. Some of the mothers that were alive during the last census die before reproducing, and some of the offspring that are born die before they can be counted in the next census [8]. Data from April sampled from 2002 to 2004 were used in demographic analysis. A correlation analysis was performed among demographic parameters in order to know relationships. Simulation model To simulate deterministic and stochastic factors affecting howler group dynamics, the RAMAS/Metapop Program [8] was used. The model used in the simulations considers a transition matrix as proposed by Caswell [49], which is a variation of the Lefkovitch [50] stage-structured model. In this matrix the population is coarsely divided into three stage classes: infants, juveniles, and adults. The matrix considers the probability that an individual remains in the same stage class from one year to the next. To simulate the number of infants, juveniles, and adults that could be expected in the following year [8], we used the equation: 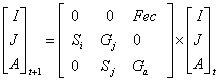
Eq. 1 In population ecology the finite growth rate (Λ) is calculated as the dominant eigenvalue of the stage matrix [8], and we used RAMAS/Metapop Program to estimate it. Robinson and Redford [51] give the maximum finite growth rate (Λ max) for Alouatta palliata as 1.162 [see 52]. Note that Λ = er where r is the instantaneous population growth rate. When value of Λ is greater than 1 the population increases in size, when Λ is lower than 1 the population declines, and when Λ is equal to 1 the population is stable. We used a density-dependent model in population trend simulations, taking into account the Ricker equation [52] in which the finite growth rate depends on abundance, fecundity, and survival rates, and carrying capacity (K) (Table 1). In population ecology K is the population size above which the population growth tends to decline [8]. However, estimation of K is difficult because it is an estimation of the maximum number of individuals per surface that the habitat can support according to available food resources. In fact, this parameter varied depending on the regional climatic conditions, local habitat composition and structure, and annual and stational variations [8]. As an indirect measure of K, the density in a low disturbed populations could be used. Because we did not find a quantitative estimation of carrying capacity for Alouatta, in this study we define K as the maximum density found in the fragments, which corresponds to 1.4 individuals/ha. This is a relaxed assumption and the consequence in the simulation is the possibility that more individuals could inhabit fragments. The general results and conclusions were not affected by this assumption since it is a constant number affecting similarly all fragments. In order to simulate environmental stochasticity, two types of catastrophes were considered: hurricanes and diseases. In Los Tuxtlas, the climatic conditions are influenced partly by tropical hurricanes; however, very few of them had a considerable impact in this region [A.Estrada, personal communication]. In previous PVA for A. p. mexicana, Rodríguez-Luna et al. [18] used an annual probability of 0.1, with a negative impact of 10% in the reproduction and 30% in the survival. In our analysis we suppose that hurricanes diminish the carrying capacity because they affect directly the canopy tree structure. Thus, in this study we considered an annual probability incidence of 0.1 with a reduction of 30% on K. With respect to diseases as a catastrophic event, it has been shown that this is a non-frequent event but one that could have a devastating effect on the populations of howler monkeys [36]. We used data of Rodríguez-Luna et al. [18] to simulate this event. In the case of diseases a probable annual impact of 0.01 was considered, resulting in a 40% reduction on reproduction and a 60% reduction of survival rate.
Simulated scenarios In this study we performed a PVA simulating two possible scenarios: "isolated populations" and "patchy population or metapopulation." In the first scenario, we assumed that occupied fragments were totally isolated from other occupied fragments. Consequently, the finite population growth rate is only affected by particular density-dependent processes occurring in each fragment. The simulation was performed using the specific transition matrix obtained for each group in Equation 1. Simulations were only performed in those groups where it was possible to estimate all elements of the matrix during the study period 2002-2004. In fragments occupied by adult individuals of both sexes, but not immatures, simulations were not performed. There was also no simulation in those fragments occupied by only one solitary individual or individuals of the same sex. In the metapopulation scenario we assumed that all groups and solitary individuals inhabiting different fragments were affected by similar environmental conditions, thus assuming complete dispersal between fragments. To simulate we used the average vital rates estimated for all groups. Thus an average transition matrix was obtained and substituted in Equation 1. In this case, all occupied fragments (by group or solitaries) were analyzed. In both simulated scenarios the forecast-predicted variables were the expected number of individuals and the probability of extinction of selected howler monkeys groups over the next 30 years. A non-linear regression model was used to explore the relationships between fragment size and probability of extinction in each scenario. Results Fragment occupation In the studied landscape, 75 to 77 howler monkeys inhabited 17.5% of fragments (19 out of 92 fragments; 348 ha of total forest area). Nine fragments were each inhabited by a separate group; four fragments were used by one group; five fragments were each inhabited by a separate solitary male; and one fragment was occupied by two males (Appendix 1). Some relatively large fragments (F36, F101 and F37) were occupied only by solitary individuals; while a small fragment (F15 = 11.8 ha) was occupied by the largest group (N = 14). Consequently, the number of individuals per group was not significantly related to the size of the fragment (Fig.2a, r2 = 0.04, P = 0.59). The average population density was 0.53 ± 0.48 individuals/ha (range 0.10 to 1.42 ind/km2, Table 1). As fragment size decreased, the density increased. In fact, the density showed a significant non-linear relationship with the fragment size (Fig. 2b, r2 = 0.70, P = 0.03). 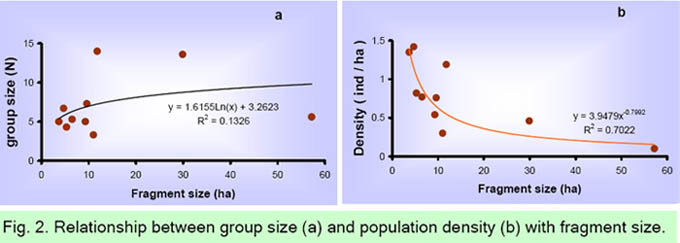
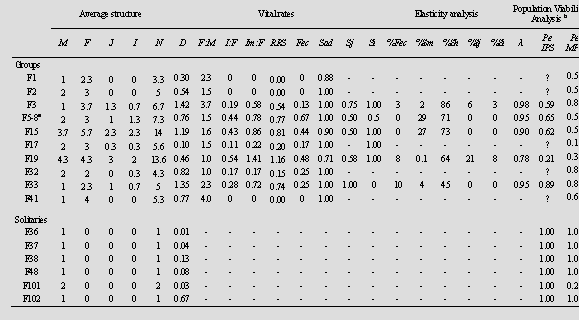
Inter-annual and average age and sex structure of each group are presented in Appendix 1 and Table 1, respectively. The average group size was 7.0 ± 3.8 (range 3 to 14) individuals, including mean 2.0 ± 1.2 adult males, 3.3 ± 1.1 adult females, 0.9 ± 1.1 juveniles, and 0.8 ± 0.8 infants. The adult sex ratio was 2.04 ± 1.05 females per male; while the ratio of infants per adult female was 0.22 ± 0.20, and immatures per adult female 0.47 ± 0.47. Group size was positively related to the number of adult females (r2 = 0.72, P = 0.002), and the number of immatures (r2 = 0.90, P = 0.0001). Consequently, the number of adult females related significantly to the number of juveniles (r2 = 0.50, P = 0.02). Vital rates The average fecundity rate was 0.24 ± 0.23 infants per female, while the relative success in reproducing (RRS) was 0.44 ± 0.42 (Table 1). In three fragments (F1, F2 and F41), no infants or juveniles were registered and the fecundity rate and RRS were therefore zero. A significant non-linear relationship between the group size and fecundity rate (Fig. 3a, r2 = 0.41, P = 0.04) and RRS (r2 = 0.62, P = 0.006); and between the number of females per group and fecundity rate (Fig. 3b, r2 = 0.75, P = 0.13) and RRS (r2 = 0.95, P = 0.02), was found. The survival rate of adults from one year to the next was 0.95 ± 0.10, juveniles 0.67 ± 0.21, and infants 0.75 ± 0.42 (Table 1). The elasticity analysis showed that the survival of adult females contributed 45% to 86% of the group growth rate (Table 1), while the survival of adult males, juveniles, and infants contributed 2-29%, 0-21% and 0-8%, respectively. The contribution of fecundity rate to population growth was 0 to 10%. 
In the isolated populations scenario, the simulated 30-year trends for the five groups with at least one immature, are presented in Fig. 4a. All groups are predicted to decline in numbers except for F19 which is predicted to increase slightly. From an initial 12 individuals in F19 we could expect a slight increase to 18 individuals at the end of the 30 years simulated. The carrying capacity could limit the growth of this group since it inhabits a medium size fragment (29.9 ha). The tendency for the other four groups is to diminish in number. In the metapopulation scenario, trends for each of the 10 groups with at least one adult male and one adult female showed that F17 was the group which tends to increase (Fig. 4b). From an initial group size of five, the group could be expected to increase to 35 individuals due to the large size of this fragment (57.2 ha). The expectation of F19 is to maintain approximately the same initial number of individuals. The tendency for the other eight groups is to diminish in number. A comparision of a total population change indicated that in the isolated populations scenario the expected number of individuals decreased, while in the metapopulation scenario no change is expected in the total number of indivuals (Fig. 5). In both the isolated populations (r2 = 0.91, P = 0.01) and metapopulation (r2 = 0.97, P = 0.01) scenarios, the probability of extinction was significantly related to the fragment size according to an exponential model (Fig. 6). As fragment size decreased, the probability of extinction increased exponentially. Fragment size less than15 ha had a 60% or more extinction probability. The largest fragments in the studied landscape (F36 = 75.5 ha, F101 = 71.0 ha, and F37 = 32.6 ha) were inhabited by solitary males; thus the contribution of these fragments to population viability is null in the actual situation. 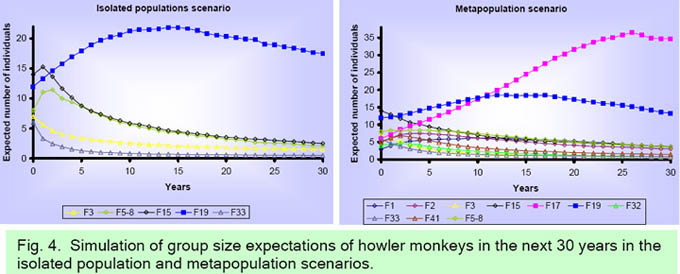
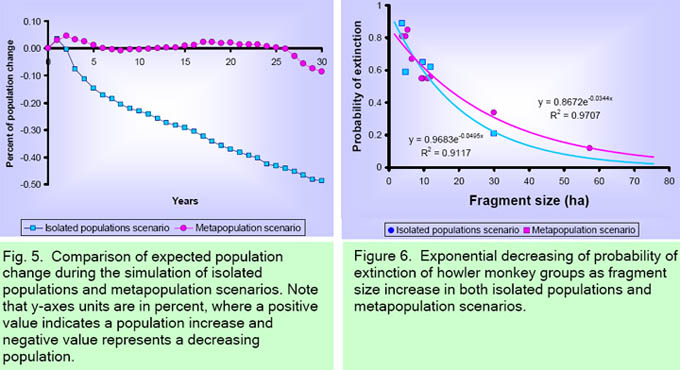
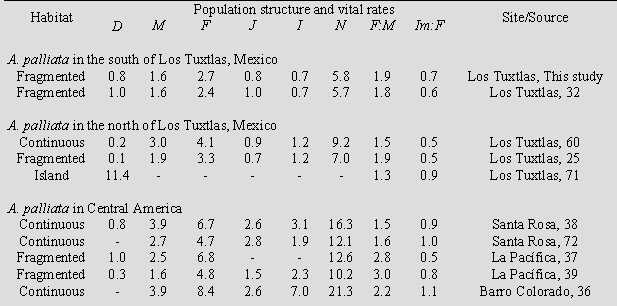
Population persistence Our PVA suggests that the probability of extinction increases exponentially as fragment size decreases. A 60% of extinction probability was estimated when fragment size is lower than 15 ha. Evidence from several primate species, including howler monkeys, suggests that<10-ha fragments have little probability of being occupied [63,64]. For instance, Cowlishaw and Dunbar [10] reported that extinction rates of three primate species increased sharply when primates inhabited < 10-ha forest fragments in the Tana River, Kenya. In Manaus, Brazil, Gilbert [63] found higher numbers of primate species and groups in 10-ha and 100-ha fragments than in 1-ha fragments. Small forest fragments support lower number of individuals [25,27,29], and as fragment size decreases the habitat quality decreases also [28,65]. In consequence, even though howler monkeys have the ability to live in small fragments for several years, our PVA suggests that viability of local population increases as fragment size and quality increase. The comparison of isolated populations and metapopulation scenarios showed that in the second the overall expected population change could be lower, due to the possibility of dispersal among fragments. Although there have been many studies on primates that inhabit fragments [11], few have addressed the problem of primate population conservation from a metapopulation perspective [10,66,67]. Metapopulation theory has been the focus of much discussion in population and conservation biology in fragmented habitats [68]. The essence of the metapopulation approach is that the presence of a given species in an area depends on the balance between the rates at which local populations become extinct and those at which new ones are established by migrants from other populations in the landscape. In consequence, metapopulations exist as various local populations within a fragmented system surrounded by a matrix. According to predictions based on metapopulation theory, if the landscape has been so severely transformed by deforestation that the number, size, quality, and connectivity of fragments are all quite low, the probability of persistence on a regional scale will decrease due to limited fragment occupation and reduced colonization of empty fragments [68]. This type of scenario clearly indicates the pressing need for a proposal of measures to mitigate and reverse fragmentation. Implications for Conservation Our PVA was based on the analysis of a single landscape and three years of census; in consequence it is necessary to continue monitoring for several years in order to have more precise estimates of demographic paramters [15]. However, PVA simulation suggests that the howler monkey population in this highly fragmented landscape is at a high local extinction risk, and conservation strategies from a metapopulation perspective are urgently required. The strategy must deal with the protection of large, occupied fragments, avoiding further loss of area and must implement habitat restoration programs. We suggest two strategies for conserving howler monkeys in the study landscape. The first is to protect priority fragments, that is, those that maintain the largest groups of howlers and that have the greatest surface area, connectivity, and habitat quality. The second is to develop reforestation strategies in order to recover and restore habitat by increasing the area of certain occupied fragments and by establishing corridors and/or stepping stones. PVA simulation suggests conserving fragments larger than 15 ha. Large fragments can sustain a greater number of individuals due to an increase in carrying capacity, decreasing the likelihood of local extinction. Several studies have illustrated that an increase in the area and number of protected sites is vital to conserving endangered populations. For example, Gilbert [63] suggested that areas larger than 100 ha are necessary for the conservation of six primate populations, Alouatta seniculus among them, in the Amazon. Chapman et al. [67] determined that Colobus guereza populations, despite their ability to colonize and survive in altered habitats, might disappear due to the rapid habitat loss. Furthermore, Lawes et al. [69] found that Cercopithecus mitis populations are more vulnerable to extinction due to habitat loss and their limited dispersal ability, as they form a metapopulation in an imbalanced state. Harcourt [17] indicated that the main threat to mountain gorilla populations was habitat loss. For Brachyteles arachnoides, Strier [15] also found that the persistence probability after 100 years was maximized by increasing available habitat and allowing the population to expand naturally. The second strategy reflects the need to restore monkey habitat. For this, two measures are necessary: increasing the size of small (< 15 ha) fragments that are presently occupied and increasing the connectivity of fragments inhabited by monkeys. For example, according to Gilbert [63], secondary vegetation corridors are important for primate populations in Central Amazonia. Swart and Lawes [66] evaluated different management strategies designed to create corridors connecting patches in a C. mitis metapopulation and concluded that in the long run (> 200 years), corridors would increase metapopulation persistence. In particular, some data suggest that howler movements may follow a stepping stone pattern when they travel from one fragment to another [42]. For primates, a stepping stone can be a group of isolated trees, live fences, riparian zones, or remnants of arboreal vegetation and/or habitat patches that are substantially smaller than an animal's home range [70]. In a previous paper, Mandujano et al. [30] estimated the number of hectares that must be reforested in order to create a scenario in which the probability of extinction at the metapopulation level is under 1%. The total reforestation area, taking into account both increased fragment size and connectivity, could vary from 112-170 ha. Species that are particularly useful are those trees that are vital to howler monkeys as sources of food and shelter; they should also serve some use to the area's human inhabitants. The possible native species for restoration of the study landscape, could be: Ficus yoponensis Desv., Cecropia obtusifolia Bertol., Brosimum alicastrum Sw., Pseudolmedia oxyphyllaria Donn Smith, Dialium guianense (Aubl.) Sandwith, Poulsenia armata (Miq.) Standl., Spondias mombin L., Nectandra ambigens (Blake) Allen, and Bursera simaruba (L.) Sarg. The majority of these tree species are also used by local inhabitants for various purposes (e.g., construction, medicinal, ornamental, forage, etc). In conclusion, our results stress the urgency of implementing conservation approaches in which increases in area of habitat fragments and connectivity are of fundamental importance for persistence of primates in the landscape. In this scenario it would be important to involve the local communities. In an on-going study we are evaluating the feasible of restoration programs considering the local human perspective. Briefly, local people are interested in conservation programs because they perceive the usefulness of the biodiversity in the fragments [Bernal Robles, personal communication]. However, socio-cultural and economic restrictions could limit any restoration program if local people don't obtain some immediate economic benefit. The negative experience of local people with previous government forestry programs has resulted in reservations in their responses to further initiatives. However, emphasizing the possible ecological services that conservation and expansion of area of forest fragments would have in capturing water, decreasing erosion, and enhancing availability of medicinal, alimentary, ornamental and woody species reservoirs, among other benefits, are aspects that are easily understood by local rural inhabitants [33]. The parallel implementation of environmental education programs would help in raising awareness among local people regarding the importance of preserving the primates in their forests. Acknowledgments We would like to thank M. Gutierrez, A. Cuarón, E. J. Naranjo, M. Equihua, and F. Garcia-Orduña, and L. G. Luecke for their comments- Thanks are also due the Consejo Nacional para la Ciencia y Tecnología and the American Society of Primatologists and Primate Conservation, Inc., who provided financial support for this study. The Departamento de Biodiversidad y Ecología Animal of the Instituto de Ecología A. C. additionally supported this project. We are grateful to three anonymous reviewers for constructive comments to improve this paper. References [1] Boyce, M.S. 1992. Population viability analysis. Annual Review of Ecology and Systematics 23: 481-506. [2] Beissinger, S.R., and D.R McCullough (eds.). 2002. Population Viability Analysis. The University of Chicago Press, Chicago, Il. [3] Morris, W.F., and D.F. Doak. 2002. Quantitative Conservation Biology: Theory and Practice of Population Viability Analysis. Sinauer Associates Inc., Sunderland, MA. [4] Reed.J.M., L.S. Mills, J.B. Dunning Jr., E.S. Menge, K.S. McKelvey, R. Frye, S.R. Beissinger, M.-C. Anstett, and P. Miller. 2002. Emerging issues in population viability analysis. Conservation Biology 16: 7-19. [5] Noon, B., F. Sampson, and N. Johnson. 2000. Population viability analysis: general principles and applications. Resource Notes 30: 1-2. [6] Beissinger, S.R., and M.I. Westphal. 1998. On the use of demographic models of population viability in endangered species management. Journal of Wildlife Management 62: 821-841. [7] Ralls, K., S.R. Beissinger, and J.F. Cochrane. 2002. Guidelines for using population viability analysis in endangered-species management. In S.R. Beissinger and D.R. McCullough (eds.), Population Viability Analysis, pp. 521-550, The University of Chicago Press, Chicago. [8] Akçakaya, H.R. 2002. RAMAS Metapop: Viability analysis for stage-structured Metapopulations (version 4.0). Applied Biomathematics, Setauket, New York. [9] Burgman, M., and H. Possingham. 2000. Population viability analysis for conservation: the good, the bad and the undescribed. In A.G. Young and G.M. Clarke (eds.), Genetics, Demography and Viability of Fragmented Populations, pp. 97-112, Cambridge University Press, Cambridge, UK. [10] Cowlishaw, G., and R. Dunbar. 2000. Primate Conservation Biology. Chicago University Press, Chicago. [11] Marsh, L.K. (ed.). 2003. Primates in Fragments, Ecology and Conservation. Kluwer Academic / Plenum Publishers, New York. [12] Caughley, G. 1994. Directions in conservation biology. Journal of Animal Ecology 63: 215-244. [13] Caswell, H. 2000. Prospective and retrospective perturbation analyses: their roles in conservation biology. Ecology 81: 619-627. [14] Kinnaird, M.F., and T. G. O'Brien. 1991. Viable populations for an endangered forest primate, the Tana River crested mangabey (Cercocebus galeritus). Conservation Biology 5: 203-213. [15] Strier, K.B. 2000. Population viabilities and conservation implications for muriquis (Brachyteles arachnoides) in Brazil's Atlantic Forest. Biotropica 32: 903-913. [16] Rylands, A.B. 1993. Population viability analyses and the conservation of the lion tamarins, Leontopithecus of south-east Brazil. Primate Conservation 14/15: 34-42. [17] Harcourt, A.H. 1995. Population viability estimates: Theory and practice for a wild gorilla population. Conservation Biology 9: 134-142. [18] Rodríguez-Luna, E., L. Cortes-Ortiz, P. Millar, and S. Ellis. 1996. Population and habitat viability assessment for the mantled howler monkey (Alouatta palliata mexicana). IUCN/SSC Conservation Breeding Specialist Group, Appley Valley, Minnesota. [19] Ballou, J.D., R.C. Lacy, D.G. Kleiman, A.B. Rylands, and S. Ellis. 1998. Leontophitecus II: The second population and habitat viability assessment for lion tamarins (Leontophitecus). IUCN/SSC Conservation Breeding Specialist Group, Apple Valley, MN. [20] Dobson, A.P., and A.M. Lyles. 1989. The population dynamics and conservation of primate populations. Conservation Biology 3: 362-380. [21] de Kroon, H., J. van Groenendael, and J. Ehrlén. 2000. Elasticities: a review of methods and model limitations. Ecology 81: 607-618. [22] Akkoc, C.C., and L.E. Williams. 2005. Population modeling for a captive squirrel monkey colony. American Journal of Primatologist 65: 239-254. [23] Dirzo, R., and M.C. Garcia. 1992. Rates of deforestation in Los Tuxtlas, a neotropical area in Veracruz, Mexico. Conservation Biology 6: 84-90. [24] Cuarón, A.D., P.C. de Grammont, L. Cortés-Ortiz, G. Wong, and J.C. Serio-Silva. 2003. Alouatta palliata ssp. mexicana. In 2007 IUCN Red List of Threatened Species. <www.iucnredlist.org [25] Estrada, A., and R. Coates-Estrada. 1996. Tropical rain forest fragmentation and wild populations of primates at Los Tuxtlas, Mexico. International Journal of Primatology 5: 759-783. [26] Estrada, A., S. Juan-Solano, T. Ortiz-Martinez, and R. Coates-Estrada. 1999. Feeding and general activity patterns of a howler monkey (Alouatta palliata) troop living in a forest fragment at Los Tuxtlas, Mexico. American Jounral of Primatology 48: 167-183. [27] Arroyo-Rodríguez, V., S. Mandujano, and J. Benítez-Malvido. 2007. Landscape attributes affecting patch occupancy by howler monkeys (Alouatta palliata) at Los Tuxtlas, Mexico. American Journal of Primatology 69: 1-12. [28] Arroyo-Rodríguez, V., S. Mandujano, J. Benítez-Malvido, and C. Cuende-Fanton. 2007. The influence of large tree density on howler monkey (Alouatta palliata mexicana) presence in very small rainforest fragments. Biotropica 39: 760-766. [29] Cristobal-Azkarate, J. , J.Vea, N. Asensio, and E. Rodriguez-Luna. 2005. Biogeographical and floristic predictors of the presence and abundance of mantled howlers (Alouatta palliata mexicana) in rainforest fragments at Los Tuxtlas, Mexico. American Journal of Primatology 67: 209-222. [30] Mandujano, S., L.A. Escobedo-Morales, R. Palacios-Silva, V. Arroyo- Rodríguez, and E.M. Rodríguez-Toledo. 2006. A metapopulation approach to conserving the howler monkey in a highly fragmented landscape in Los Tuxtlas, Mexico. In P. Garber, A. Estrada, M. Pavelka, and L. Luecke (eds.), New Perspectives in the Study of Mesoamerican Primates: Distribution, Ecology, Behavior and Conservation, pp. 513-538. Springer, New York. [31] Soto, A., and L. Gama. 1997. Climas. In E. Gonzalez-Soriano, R. DirzoEstrada, and R.C. Vogt (eds.), Historia Natural de Los Tuxtlas, pp. 7-23. UNAM, México D. F. [32] Rodríguez-Luna, E., F. García-Orduña, G. Silva-López, and D. Canales-Espinosa. 1987. Primate conservation in Mexico. Primate Conservation 8: 114-118. [33] Silva-López, G., and E. Portilla-Ochoa. 2002. Primates, lots and forest fragments: ecological planning and conservation in the Sierra de Santa Marta, México. Neotropical Primates 10: 9-11. [34] Rodríguez-Toledo, E.M., Mandujano, S., and García-Orduña, F. 2003. Relationships between characteristics of forest fragments and howler monkeys (Alouatta palliata mexicana) in southern Veracruz, México. In: Marsh, L. K. (ed.), Primates in Fragments: Ecology and Conservation. Kluwer Academic /Plenum Press, New York, pp. 79-97. [35] Bicca-Marques, J. C. 2003. How do howler monkeys cope with habitat fragmentation? In: Marsh, L. K. (ed.), Primates in Fragments: Ecology and Conservation, Kluwer Academic/Plenum Publishers, New York, NY, pp. 283-303. [36] Milton, K. 1982. Dietary quality and demographic regulation in a howler monkey population. In E.G. Leigh, A.S. Rand, and D.M. Windsor (eds.), The Ecology of a tropical forest: Seasonal rhymes and long-term changes, pp. 273-289. Smithsonian Institution Press, Washington. [37] Clarke, M.R., and E.L. Zucker. 1994. Survey of the howling monkeys population at La Pacifica: a seven-year follow-up. International Journal of Primatology 15: 61-73. [38] Fedigan, L.M., L.M. Rose, and R.M. Avila. 1998. Growth of mantled howler groups in a regenerating Costa Rican dry forest. International Journal of Primatology 19: 405-432. [39] Clarke, M. R., D.A. Collins, and E.L. Zucker. 2002. Responses to deforestation in group of mantled howlers (Alouatta palliata) in Costa Rica. International Jorunal of Primatology 23: 365-381. [40] Clarke, M.R., C.M. Crockett, E.L. Zucker, and M. Zaldivar. 2002. Mantled howler population of Hacienda La Pacifica, Costa Rica, between 1991 and 1998: effects of deforestation. American Jorunal of Primatology 56: 155-163. [41] Crockett, C.M., and J.F. Eisenberg. 1987. Howlers: variations in group size and demography. In B.B. Smuts, D.L. Cheney, R.M. Seyfarth, R.W. Wrangham, and T.T. Struhsaker (eds.), Primates Societies, pp. 54-68. The University of Chicago Press, Chicago and London. [42] Glander, K.E. 1992. Dispersal patterns in Costa Rica mantled howling monkeys. International Journal of Primatology 13: 415-436. [43] Jones, C.B. 1995. Howler monkeys appear to be preadapted to cope with habitat fragmentation. Endangered Species UPDATE 12: 9-10. [44] Pope, T. R. 1992. The influences of dispersal patterns and mating system on genetic differentiation within and between populations of the red howler monkey (Alouatta seniculus). Evolution 46: 1112-1128. [45] Crockett, C.M. 1996. The relation between red howler monkey (Alouatta seniculus) troop size and population growth in two habitats. In Norconk (ed.), Adaptative Radiations of Neotropical Primates, pp. 489-510. Plenum Press, New York. [46] Crockett, C.M. 1998. Conservation biology of the genus Alouatta. Internation Journal of Primatology 19: 549-577. [47] Jones, C.B. 1996. Relative reproduction success in the mantled howler monkey: implications for conservation. Neotropical Primates 4: 21-23. [48] Estrada, A., A. Mendiza, L. Castellanos, R. Pacheco, S. Van Belle, Y. Garcia, and D. Muñoz. 2002. Population of the black howler monkey (Alouatta pigra) in a fragmented landscape in Palenque, Chiapas, Mexico. American Journal of Primatology 58: 45-55. [49] Caswell, H. 1989. Matrix population models: Construction, analysis, and interpretation. Sinauer Assocoates, Sunderland, Massachussets. [50] Lefkovitch, L.P. 1965. The study of population growth in organisms grouped by stages. Biometrics 21: 1-18. [51] Robinson, J.G., and K.H. Redford. 1986. Intrinsic rate of natural increase in Neotropical forest mammals: relationship to phylogeny and diet. Oecologia (Berlin) 68: 516-520. [52] Ricker, W.E. 1975. Computation and Interpretation of biological statistics of fish populations. Bulletin 191 of the Fisheries Research Board of Canada, Ottawa. [53] Van Belle, S., and A. Estrada. 2006. Demographic features of Alouatta pigra populations in extensive and fragmented forests. In P. Garber, A. Estrada, M. Pavelka, and L. Luecke (eds.), New Perspectives in the Study of Mesoamerican Primates: Distribution, Ecology, Behavior and Conservation, pp. 121-142. Springer, New York. [54] Chapman, C.A., and S.R. Balcomb. 1998. Population characteristics of howlers: ecological conditions or group history. International Journal of Primatology 19: 385-403. [55] Milton, K., and M.E. Hopkins. 2006. Growth of reintroduced spider monkey (Ateles geoffroyi) population on Barro Colorado Island, Panama. In P. Garber, A. Estrada, M. Pavelka, and L. Luecke (eds.), New Perspectives in the Study of Mesoamerican Primates: Distribution, Ecology, Behavior and Conservation, pp. 417-4435. Springer, New York. [56] Crockett, C.M., and T.R. Pope. 1993. Consequences of sex differences in dispersal for juvenile red howler monkeys. In M.E. Pereira, and L.A. Fairbanks (eds.), Juvenile Primate: Life History, Development, and Behavior, pp. 104-118. Oxford University Press, New York. [57] Agoramoorthy, G., and R. Rudran. 1993. Male dispersal among free-ranging red howler monkeys (Alouatta seniculus) in Venezuela. Folia Primatologica 61: 92-96. [58] Calegaro-Marques, C., and J.C. Bicca-Marques. 1996. Emigration in a black howling monkey group. International Journal of Primatology 17: 229-237. [59] Jones, C. B. 1999. Why both sexes leave: effects of habitat fragmentation on dispersal behavior. Endangered Species UPDATE 16: 70-73. [60] Estrada, A. 1982. Survey and census of howler monkeys (Alouatta palliata) in the rain forest of Los Tuxtlas, Veracruz, Mexico. American Jorunal of Primatology. 2: 363-372. [61] Mandujano, S., L.A. Escobedo-Morales, and R. Palacios-Silva. 2004. Movements of Alouatta palliata among forest fragments in Los Tuxtlas, Mexico. Neotropical Primates 12: 126-131. [62] With, K. A. 2004. Metapopulation dynamics in highly fragmented landscapes. Pp. 23-44, in Hanski, I. and Gaggiotti, O. E. (eds.), Ecology, Genetics, and Evolution of Metapopulations, Elsevier Academic Press, Burlington, MA. [63] Gilbert, K. A. 2003. Primates and fragmentation of Amazon forest. In: L. K. Marsh (Ed.). Primates in Fragments: Ecology and Conservation, Kluwer Academic/Plenum Publishers, New York, pp. 145-157. [64] Mandujano, S., and A. Estrada. 2005. Detection of area and isolation distance thresholds for forest fragments occupation by howler monkeys, Alouatta palliata, in Los Tuxtlas, Mexico. Universidad y Ciencia II: 11-21. [65] Arroyo-Rodríguez, V., and S. Mandujano. 2006. Forest fragmentation modifies habitat quality for Alouatta palliata. International Journal of Primatology 27: 1079-1096. [66] Swart, J., and Lawes, M. J. (1996). The effect of habitat patch connectivity on samango monkey (Cercopithecus mitis) metapopulation persistence. Ecological Modeling 93: 57-74. [67] Chapman, C. A., Lawes, M. J., Naughton-Treves, L., and Gillespie, T. 2003. Primate survival in community-owned forest fragments: Are metapopulation models useful amidst intensive use? In: Marsh, L. K. (ed.), Primates in Fragments: Ecology and Conservation, Kluwer Academic/Plenum Publishers, New York, NY, pp. 63-78. [68] Hanski, I. 1999. Metapopulation Ecology. Oxford University Press, Osney Meads, UK. [69] Lawes, M. J., Mealin, P. E., and Piper, S. E. (2000). Patch occupancy and potential metapopulation dynamics of three forest mammals in fragmented afromontane forest in South Africa. Conservation Biology 14: 1088-1098. [70] Palacios-Silva, R., and S. Mandujano. 2007.Conectividad de parches de hábitat para los primates en un paisaje altamente fragmentado en el sureste de México. In: J. Saénz y C. Harvey (eds.), Evaluación y Conservación de la Biodiversidad en Paisajes Fragmentados de Mesoamérica. Universidad Nacional (EUNA), Costa Rica. [71] Carrera-Sánchez, E., G. Medel-Palacios, and E. Rodríguez-Luna. 2003. Estudio Poblacional de Monos Aulladores (Alouatta palliata mexicana) en la Isla Agaltepec, Veracruz, México. Neotropical Primates 11: 176-180. [72] Fedigan, L.M., and K. Jack. 2001. Neotropical primates in a regenerating Costa Rican dry forest: A comparison of howler and capuchin population patterns. International Jorunal of Primatology 22: 689-713. Appendix 1. Annual abundance and group composition of howler monkeys, and of forest fragment characteristics in the study area. M = adult males, F = adult females, J = juveniles, I = infants, Tot = total of individuals a during 2001 specific data (sex, age) were not observed in detail in each group by Rodríguez-Toledo et al. [34] b fragment 19 inhabited 3 groups, c fragments 5 to 8 were used by one group, therefore the size is the sum of each fragment and isolation is the mean distance, d fragment 101 and 102 was sampled only once in 2003, thus is lacking precise data.
Comments Reader comments are generally moderated. If you find something inappropriate, please contact Tropical Conservation Science. The opinions expressed in reader comments are those of the author only, and do not necessarily reflect the opinions of other authors or Tropical Conservation Science. |
 |
Tropical Conservation Science is an open-access e-journal that publishes research relating to conservation of tropical forests and other tropical ecosystems.
Volume 1: Issue 1 Table of Contents Articles PDF version General interest review All issues Mar 2008 Jun 2008 Sep 2008 Dec 2008 Mar 2009 Jun 2009 Sep 2009 Dec 2009 Mar 2010 Jun 2010 Sep 2010 Dec 2010 Mar 2011 Jun 2011 Sep 2011 Dec 2011 Mar 2012 Jun 2012 Sep 2012 Dec 2012 Mar 2013 Jun 2013 Aug 2013 Sep 2013 Nov 2013 Dec 2013 Mar 2014 Jun 2014 Sep 2014 Dec 2014 Mar 2015 Jun 2015 Sep 2015 Dec 2015 Mar 2016 Jun 2016 Most downloaded 2008 2009 2010 2011 2012 All time ADVERTISEMENT SEARCH  This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
|
About | Privacy Copyright mongabay.com 2008-2014 |