Research Article
Some aspects of seed dispersal effectiveness of golden lion tamarins (Leontopithecus rosalia) in a Brazilian Atlantic forest
Marina Janzantti Lapenta 1,2,3* and Paula Procópio-de-Oliveira 1,3
1- Golden Lion Tamarin Association (Associação Mico-Leão-Dourado-AMLD),
2- Ecology Department, IB-University of São Paulo (USP)
3- Instituto Pri-Matas para a Conservação da Biodiversidade Caixa Postal 3304, Savassi, Belo Horizonte, MG. CEP: 30140-970 BRASIL
* Corresponding author email: [email protected]
Abstract
The effectiveness of a seed disperser is assessed by the quantity (number of visits to fruiting trees and number of seeds dispersed) and quality of dispersal (seeds passed through the gut unharmed and how and where the seeds are dispersed). This is the first study to examine quantitative and qualitative aspects of seed dispersal by golden lion tamarins (Leontopithecus rosalia). The study was conducted from December 1998 to December 2000 and from April 2003 to March 2004 in the União Biological Reserve, Brazil. We marked 1,185 fruiting trees visited by tamarins and collected 1039 feces with seeds. About 76% of feces deposited by L. rosalia did not differ from the spatial distribution of plants of the same species. The mean time of gut retention was 1:14 h, and the mean distance of dispersal was 105 m. Given the effective role of L. rosalia as seed dispersers, their presence in the Atlantic forest is important for the regeneration of the forest.
Key Words: Dispersal distance, gut passage, seed dispersal, golden lion tamarins, Leontopithecus rosalia.
Resumo
A eficiência de um dispersor de sementes é verificada pela quantidade (número de visitas às árvores frutificadas e o número de sementes dispersadas) e qualidade da dispersão (sementes que passam intactas pelo trato digestório e como e onde são dispersas). Este é o primeiro estudo a examinar aspectos quantitativos e qualitativos da dispersão de sementes por micos-leões-dourados (Leontopithecus rosalia). O estudo foi conduzido de dezembro de 1998 a dezembro de 2000, e de abril de 2003 a março de 2004, na Reserva Biológica União, Brasil. Nós marcamos 1185 árvores frutificadas visitadas pelos micos e coletamos 1039 fezes com sementes. Cerca de 76% das fezes depositadas por L. rosalia não diferem da distribuição espacial das plantas das mesmas espécies. O tempo médio de retenção no trato digestório foi de 1:14 h, e a média da distância de dispersão foi de 105 m. Devido à eficiência no papel de L. rosalia como dispersor de sementes, sua presença na Mata Atlântica é importante para a regeneração da floresta.
Palavras-chave: Distância de dispersão, passagem pelo trato digestório, dispersão de sementes, mico-leão-dourado, Leontopithecus rosalia.
|
Received: 8 April, 2008, 11 May, 2008, Published: 9 June, 2008
Copyright: © 2008 Lapenta, M. J. and Procópio-de-Oliveira, P. This is an open access paper. We use the Creative Commons Attribution 3.0 license http://creativecommons.org/licenses/by/3.0/us/ .The license permits any user to download, print out, extract, archive, and distribute the article, so long as appropriate credit is given to the authors and source of the work. The license ensures that the published article will be as widely available as possible and that your article can be included in any scientific archive. Open Access authors retain the copyrights of their papers. Open access is a property of individual works, not necessarily journals or publishers.
Cite this paper as: Lapenta, M. J. and Procópio-de-Oliveira, P. 2008. Some aspects of seed dispersal effectiveness of golden lion tamarins (Leontopithecus rosalia) in a Brazilian Atlantic forest . Tropical Conservation Science Vol.1 (2):122-139. Available online: tropicalconservationscience.org
Introduction
The transport from the parental plant to a site where a seed can germinate is one of the main aspects of seed dispersal. According to the Janzen-Connell Model [1-2], seeds have a higher probability of survival if dispersed away from parental trees and in habitat suitable for germination. The escape hypothesis [3] states that dispersed seeds have a higher probability of survival, avoiding predation, diseases and intraspecific competition, dangers considered strongest near the parental tree. If the tree species is a colonizer and depends on resources that are randomly distributed, like light penetrating gaps in the canopy, it will have a low probability of germination and recruitment in high densities under the parental tree [4-6].
The effectiveness of a frugivorous species as a seed disperser refers to its capacity to deliver seeds to safe sites, resulting in survival and germination [7-10]. The effectiveness of a species as an agent of seed dispersal is influenced by its morphology, physiology, and behavior [11], and can be assessed by the quantity and quality of dispersal. Quantity refers to the number of visits and the number of seeds dispersed per visit to a tree, whereas the quality refers to the place where the seeds are deposited and how they are dispersed [12].
Many studies concerned with seed dispersal by primates have not considered the quality of the sites where the seeds are deposited [13-15], and for many plant species, the place of deposition cannot be precisely defined as suitable or unsuitable for establishment and survival of seedlings [16]. In this study, we considered safe sites for germination to be those microhabitats where plants consumed by tamarins are currently distributed, but the majority of seeds dispersed by primates on forest sites are killed by seed predators or moved by secondary dispersers. Nevertheless the place where the seeds were removed by secondary dispersers may be more adequate to establishment and survival than the place of primary dispersal [8].
The distance of seed dispersal depends on animal movement patterns through the habitat and time that seeds remain in the gut or are carried by them [17-19]. Many factors may influence the time of seed retention in the gut, e.g., the size and weight of seeds, seed volume, diet quality, and the morphological and physiological characteristics of the species and individuals [10, 14, 19-22].
Callitrichids have not figured prominently in studies of seed dispersal [10, 23-28] and more studies with seed dispersal by these small primates are therefore needed to understand their potential for contributing to the natural regeneration of undisturbed and disturbed forests [24].
This study is the first to consider some quantitative and qualitative aspects of seed dispersal by L. rosalia and the role of the species as an effective seed disperser in patches of Atlantic Forest in the state of Rio de Janeiro. The aims were to determine: 1) the habitat occurrence of each feeding tree visited by the tamarins, 2) the number of seeds per defecation; 3) whether GLT feces deposition differs from the spatial distribution of plants; 4) the distance that seeds are dispersed from the parental tree; and 5) the relationship between gut passage time of the seeds and dispersal.
Methods
Study site
The study was conducted in the União Biological Reserve (22°27'36"S, 42°02'15"W), in the municipalities of Casimiro de Abreu and Rio das Ostras, in the state of Rio de Janeiro-Brazil (Fig. 1). The Reserve, administered by the Brazilian Institute for the Environment (IBAMA), is an area of 3,121.2 ha with 2,400 ha of forest, divided by Federal Highway BR 101.
There was no native golden lion tamarin (Leontopithecus rosalia) population in the União Farm, despite being located within the natural range of the species. The formation of this new population started with the rescue of six wild tamarins groups from small and isolated forest fragments and their translocation to the new site from 1994 to 1997 [29-30]. In 1998, the farm was transformed into the União Biological Reserve (UBR), protecting the population of GLT. In 2006 (after 12 years of first translocation ), the UBR population was formed by 220 tamarins distributed in 30 groups [31].
The climate in the region is hot and humid with a defined seasonality [32]. The dry season occurs from April to September, and the wet season from October to March. The mean annual rainfall is 1,678 ± 305 mm with maximum temperature averaging 27.9 ± 4.3ºC and minimum temperature averaging 20.3 ± 3.3ºC [33]. Kierulff [34] distinguished three types of vegetation in the UBR, based on topography and drainage systems: 1) Swamp Forest, flooded areas, low density of vegetation in the canopy, and high density of lianas and of vegetation in the understory. Mean height of trees in the canopy 12,9 ± 4,2m (ranging from 6.2 to 25 m) (Fig.2a); 2) Lowland Forest, seasonal standing water, numerous epiphytes and mean height of trees in the canopy 19,2 ± 4,7m (ranging 9 to 29.8 m); 3) Hill Forest, low density of epiphytes, high density of vegetation in the canopy, and mean height of trees in the canopy 22,4 ± 3,9m (ranging 14.4 to 32.8 m) (Fig. 2b). The Swamp and Lowland forests were difficult to differentiate in the field during dry months and the analyses were done joining these two types of vegetation.
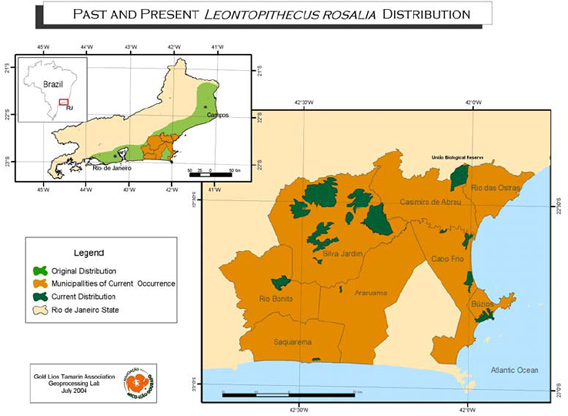
Fig. 1. The original and current distribution of the golden lion tamarin (Leontopithecus rosalia) in the Rio de Janeiro State, Brazil and study area (União Biologica Reserve).

Fig. 2. Habitat in the União Biological Reserve, RJ-Brazil. (a) Swamp Forest, ( b) Hill Forest. © MJ Lapenta.
Study species
Leontopithecus rosalia (Fig. 3) is an endemic primate of the coastal Atlantic Forest currently restricted to eight municipalities in the state of Rio de Janeiro [35]. The species is ranked as "endangered" according to the IUCN Red List of Threatened Species [36]. Fragmentation and degradation of the forests formerly occupied by lion tamarins are the main reasons they are so threatened today [37]. About 20% of the original area of L. rosalia is still forested, but 60% of it is fragmented into small patches between 2.0 and 0.2 Km2 [38].

Fig. 3. Golden Lion tamarin (Leontopithecus rosalia). © L. Candisani
The golden lion tamarins (GLT) are small-bodied primates that live in cohesive groups. The mean weight calculated for the UBR population was 544.4±44.6 [31]. The diet of GLT consists of fruits, nectar, insects, and other invertebrates, small vertebrates, exudates, and fungi [39-41]. In general seeds swallowed by tamarins are elongate and besides the seed size the seed shape and the adherence of the pulp influence whether it is swallowed or not by the tamarins [42]. During the study period the mean length of daily activity in the UBR was 10.4 h, and the mean daily path traveled was 1.522 m [42-43].
A previous study of the germination of seeds dispersed by GLT concluded that they are seed disseminators due to the germination viability of some seeds of the fruits ingested, but the percentages and rate of seed germination are not altered [28]. The tamarins have an important role in other aspects of seed dispersal, such as dispersal distance and location of seed deposition.
Together with L. rosalia, other frugivorous primates occur in the UBR: the capuchin monkeys (Cebus nigritus), the howler monkeys (Alouatta guariba), and other frugivorous mammals, like the squirrels (Sciurus aestuans), south american coati (Nasua nasua), kinkajou (Potos flavus), tayra (Eira barbara), crab-eating fox (Cerdocyon thous), bats and many rodents and marsupials. There are many frugivorous birds, too (pers. obs.).
Observations of GLT
The study was conducted during two different periods, when three groups of GLT (two groups per time period) previously habituated were followed since they left the sleepping tree in the morning until the end of the day, when they went to sleep. The first study was conducted from December 1998 to December 2000, during which time the groups LB (three to six individuals) and SJ2 (six to 12 individuals) were followed for a total of 871.9 h. The second study was conducted from April 2003 to March 2004, when the groups SJ2 (eight to 13 individuals) and Geni (seven to 13 individuals) were followed during 712 h of observation. The groups LB and SJ2 were translocated to UBR in 1994 and 1995, respectively, and the Geni Group was formed in 1998 in UBR with a male and a female that migrated from different translocated groups. The groups were cohesive, and all group members were studied equally throughout the period the group was observed. The data were collected specifically to obtain information on fruit feeding and seed dispersal (each time a tamarin fed on fruits or deposited feces with seeds) and were pooled together for analysis. We recorded 1,534 feeding events.
Quantifying presence of seeds in feces
All the fruiting trees (or other plant forms) visited by GLT for fruit consuming were marked with numbered flags, and the habitat and the position (coordinates "x" and "y") were transformed to UTM and plotted on a map of the area. We considered a consumption event to be every time at least one individual of the group arrived at a plant and fed on fruits.
The feces from all group members containing ingested seeds were collected, numbered and plotted on the map of the area. It was not possible to find all feces when the group was scattered or moving very quickly. The feces without seeds were not collected, and the number of seeds in the feces was counted only for species with seeds > 3 mm length.
Deposition sites
We assumed that seeds of a fruit species would have better chances of germinating and establishing if defecated in the same habitat (with the same abiotic characteristics) where adult trees are spatially distributed. We tested whether the distribution of feces across habitat types hill, lowland, and Swamp, was significantly different from that of the feeding trees across habitat, using a Chi-square test for each tree species. We pooled the data from Lowland and Swamp into a single category, due to the difficulty of separating these habitats visually during dry months. There are more differences related to spatial distribution of the feeding resources when we compare hill forests with lowland and swamp forests. Some species ocurr exclusively in the hill forest and others are registered and spatially distributed in both lowland and swamp forest, only differing on its density [33].
Dispersal distances
The estimation of retention times and dispersal distances were calculated for all species, using the tree and feces deposit position on a map of the area (10 m of accuracy). As in McConkey [21], we only measured the dispersal distances when we were certain of the parent tree, using the interval between the feeding time and first appearance of the seeds in the feces of each species. The calculations were made with caution, through continuous observation of a group over a complete day (from sleep tree to sleep tree) and days followed over a month. On the first day, we considered only seeds defecated after one hour following the beginning of data collection to avoid counting defecated seeds ingested prior to the observation. Sometimes, however, the individuals of the group fed on many trees of the same species at the same time, or at intervals of only a few minutes, and it was impossible to calculate the time of seed passage through the gut of a frugivore because the relationship between the time of feeding and the deposited feces could not be precisely determined.
Data analysis
We used a chi-square test for each tree species to see whether the spatial distribution of feces across habitat types was significantly different from that of the feeding trees across habitat (2 x 2 Contingency Table; comparing the number of trees in each habitat versus the number of feces deposits in the same habitat). The estimation of dispersal distance was done using the Arc View 3.2 Animal Movement Analysis Extension calculating the straight line between the tree's position and feces deposit on the map. The correlations among mean size of seeds, mean time of seed retention, and dispersal distance were tested using the Spearman Correlation Coefficient [44].
Results
Fruit species used and seeds dispersed
The tamarin groups fed on 1,185 fruiting plants from 97 species (Appendix 1). During feeding the tamarins ingested the seeds of 76 species (78.3 %) and spat the seeds of 21 species (21.7%). The fruit species most visited for fruit consuming were Miconia latecrenata, Sarcaulus brasiliensis, Inga thibaudiana and Pourouma guianensis (Appendix 1) and the greatest number of fruit-consumption events occurred in Lowland and Swamp Forest (74.6%) (Fig. 4).

Fig. 4. Fruits consumed and dispersed by golden lion tamarins in the União Biologica Reserve. (a) Cecropia hololeuca, (b) Miconia latecrenata, (c) Pourouma guianensis, (d) Pouteria bangii. Photos (a), (b), (d) © M.J. Lapenta; (c) © AMLD
The GLT's feces have a lack of cohesiveness with little or no organic material. During the study, we collected 1,039 fecal samples containing seeds from 76 species, and the majority of feces were deposited on Lowland and Swamp habitat (69.4%).
The mean number of seeds per fecal sample (for all species with seeds > 3 mm length) was 3.8 ± 2.5 (range 1 to 28 seeds), but 31 percent of fecal samples contained only one seed and about 50 percent of feces contained two to four seeds. A total of 108 fecal samples with seeds from at least 46 species contained seeds from more than one species, with a maximum of three species of seeds per defecation.
Deposition sites
We compare the habitat of 1,039 feces deposits of 43 species (from the 97 fruit species consumed by tamarins) with the habitat of 982 feeding trees (Fig. 4). Individual chi-square analyses were done for the 21 species for which enough data had been collected. When data on feeding fruits and seed deposition in lowland and swamp were pooled together, the seed depositions differed from the distribuition of feeding trees for only five species (23.8%): C. hololeuca, M. latecrenata, Miconia sp.1, S. brasiliensis and Micropholis guyanensis (Appendix 1).
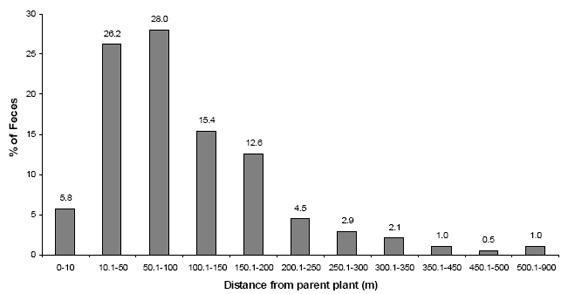
Fig. 5. Distribution of dispersal distances of golden lion tamarin feces
Dispersal distance
The tamarins defecated throughout the day. The mean time of seed retention in the gut for all ingested species was 1:14h ± 0:18h (N = 187 samples; 39 species). The minimum retention time of seeds occurred in Myrtaceae sp.1 (0:21h), and the maximum time of gut passage occurred in Hyperbaena domingensis (3:20h) (Appendix 2). The time of seed retention was not correlated with the length (N = 21; rs = 0.23; P = 0.32) nor with the diameter of seeds (N = 20; rs = 0.44; P = 0.053).
The mean distance of seed dispersal in relation to the parental tree was 105 ± 68 m, estimated for 382 defecations (57 species). The distance varied from 0 to 858 m (Appendix 2). The distance of seed dispersal was not correlated with the time of seed retention in the tamarins' guts (N = 38; rs = 0.059; P = 0.72) nor with the length (N = 23; rs = 0.23; P = 0.29) or diameter of seeds (N = 22; rs = 0.05; P= 0.81). Only 5.8 percent of the tamarins' fecal samples were located within 10 m of the parental tree (2.9% just under the feeding tree), and 314 fecal samples (82.2%) were found between 10 and 200 m (Fig. 5).
Discussion
Pattern of feces deposition and deposition sites
Feces deposition patterns of primates vary according to the species, and the deposition by GLT may be considered intermediary when compared to the clumped or scattered pattern of other primates. The seeds deposited by the tamarins are not surrounded by much organic material [10, 24 and this study], and as with the frugivore spider monkey (Ateles spp.), the feces have a lack of cohesiveness and are not compact like those of howlers (Alouatta spp.) [45-46]. Thus, it is sometimes impossible to quantify the number of seeds in feces, mainly seeds smaller than <3mm. According to Wehncke et al. [47], individuals in a group of Alouatta palliata have long gut passage time, defecate simultaneously, and the seeds are concentrated under the sleeping sites [13, 48-49]. Also the pattern of seed deposition may be related to the area. In disturbed forests the seeds defecated by A. palliata may have a clumped distribution pattern, whereas in preserved sites they are more regularly distributed [50]. The capuchin monkey (Cebus capucinus) has rapid gut passage, and the feces are scattered and deposited at large distances from the parental tree [47]. The same pattern of seed dispersal was found for muriquis (Brachyteles arachnoides) [51] and spider monkeys (Ateles spp.) [52-53], but the number of seeds dispersed by the spider monkeys under sleeping sites was greater than at in-transit sites [54]. GLT feces were not concentrated under sleeping trees like howler monkey feces (Alouatta spp.) [49,55], in nests like gorillas (Gorilla spp.) [4, 56], nor at feeding sites like gibbons (Hylobates spp.) [21]. The tamarins were observed defecating under sleeping trees, but also in other places of the forest while they were in transit. According to the Janzen-Connell model and depending on species, this should make the tamarins more effective seed dispersers, since high seed deposition under trees used repeatedly as sleeping or feeding sites on consecutive days results in low probabilities of survival compared to seeds dispersed during animal locomotion, or in nests used only once [21, 23, 57].
As in the present study, a comparison between the effectiveness of seed dispersal by Alouatta guariba and Brachyteles arachnoides found a maximum of three seed species per fecal sample [51]. However, the mean number of seeds per sample was greater than the 3.8 found in this study (means of 17.6 per feces for A. guariba and 9.9 for B. arachnoides). Besides the small size of the lion tamarins the difference in relation to A. guariba and B. arachnoides is probably due to the decreased cohesiveness of fecal material deposited by them. Studies with small tamarins found the greatest number of 13 seeds passed by a captive moustached tamarin (Saguinus mistax) and six seeds passed by a saddle-back tamarin (S. fuscicollis) [10]. Other studies with these two primates found a variation between 1 and >4,800 seeds per fecal sample (including feces with seeds <3mm not considered in the present study) [24]. In this study we did not consider the number of seeds < 3mm in feces, but we used the number of fruits of Miconia latecrenata consumed by one group of tamarins in a preview study [33] and extrapolated it to estimate the number of seeds < 3 mm ingested by the tamarins. Procópio-de-Oliveira [33] registered 3,158 fruits of M. latecrenata (with about 100 seeds per fruit) consumed by each tamarin monitored for three days per month during one year. The study group has eight adult tamarins, so we found a total of 2,526,400 seeds ingested by this group during one year. Of course it can change year by year due to fruit availability but if we consider this number, the mean number of seeds per fecal sample could be higher than the results presented in this paper.
In addition to the effect of seed passage through the gut, recruitment will only be successful if the habitat where the seed is deposited is appropriate [58]. Most tree species visited by the GLT were present in more than one habitat, but the number of defecations containing seeds of a given species deposited in each habitat did not differ from the number of plants of the same species in one of the three habitats. The limiting factor of distribution of some species may be related to other factors (light conditions, degree of canopy overlap, soil type, degree of habitat disturbance, etc.), and not only to terrain configuration or to soil humidity, as considered in this study (hilly places being drier than the lowland, and the swamp being flooded during rainy months). It was sometimes difficult to visually distinguish the habitats of lowland and swamp during the data collection (mainly in transitional environments or during periods of intense drought). Thus we performed a Chi-square test merging the data from swamp and lowland habitat. We found that for 76.2 percent of species tested, the feces were usually distributed across the habitat in a pattern similar to that of the parent trees (Table 1). To pursue this type of inquiry, more complete studies are needed to assess seed dispersal quality in terms of safe sites. This approach may consider the experimental transplanting of seeds and seedlings to different forest types, to forest gaps, and to different distances from parent trees [5-6]. Importantly, follow-up studies need to investigate the role played by secondary dispersers (e.g., rodents, ants, dung beetles, and others) on modulating post-dispersal seed and seedling fate [8, 55, 59-62].
Dispersal distance
The time of seed retention in a frugivorous species may vary significantly with the ingested fruit [14, 63]. Stevenson [19] concluded that for woolly monkeys (Lagohtrix lagothricha), small seeds remain longer in the gut than medium or large seeds. Our results indicated that for the GLT, seed size did not influence retention time, but the almost significant correlation found between retention time and seed diameter suggests that it is possible that with a larger sample size the relationship might be detected. Primates that feed mainly on fruits have a shorter gut retention time than those who also feed on leaves [19-20, 51,64]. The spider monkey (Ateles geoffroyi), a frugivorous specialist, has a digestion period of 4.4 ± 1.5 h, while the howler monkey (Alouatta palliata), which feeds mainly on leaves, has a mean period of 20.4 ± 3.5 h [65-66]. The common marmoset (Callithrix jacchus), a small neotropical primate, feeds on fruits and insects, but also on plant exudates, and has a gut passage time of 3.3 to 3.6 hours. About 60% of seeds ingested by the tamarins Saguinus spp. passed through the gut in less than three hours [10]. The GLT have rapid food passage, enabling them to process a considerable volume of food in a short amount of time, and to ingest seeds of large size. Probably the variation in gut passage is related to the time of day, number of seeds ingested or seed species, and other food ingested (insects and small vertebrates).
In this study, no correlation was found between seed passage time and seed dispersal distance. A similar result was found by Stevenson [19], studying woolly monkeys, but Garber [10] found that the passage through tamarins' digestive tract was positively correlated with the distance of dispersal from parent tree. In this study the results can be explained by the movement of the primates in the forest. Like the gibbons [20] and Gorilla spp. [67], the lion tamarins repeat visits to trees with abundant resources, during the day or on consecutive days, and sometimes short distances are traveled due to the aggregate position of fruiting species, or to the long resting periods of individuals during hot months (pers. obs.).
Seeds usually have greater chances of survival if dispersed away from the parental tree than than if deposited directly under the tree [23, 68], and the seed shadows generated by GLT were characterized by the majority of depositions far from parental trees. Seed dispersal over long distances may be important for genetic variability and survival of endangered plant species present in fragmented areas [69]. In addition, long dispersal distances may help pioneer trees invade gaps inside the forest where the densities of adults are very low [70]. But seed dispersal over long distances may be harmful for the recruitment of some species that are exclusive to specific microhabitats which may be patchily distributed across the forest, because it increases the possibility of deposition in an unfavorable habitat. In the present study, seed dispersal distances were not estimated by the most rigorous methods which include molecular paternity analyses and long focal animal sampling [19, 71], but the majority of the seeds were defecated at a distance between 10 and 100m from the parent tree, which was outside the seed shadow created by seeds dropped under the parent tree, and similar to other tamarin species (between 34 and 513m [10]. The size of the area used by the disperser may also influence the distance of dispersal [13]. In this study, we did not measure the distance of the fecal deposition in relation to other trees of the seed species present in scats. The distribution of these trees may influence survival, germination, or predation of the seeds defecated by tamarins, as may the seed shadow of the trees where the seeds were consumed.

Fig. 6. View of the União Biological Reserve. © AMLD
Implications for conservation
In the past, deforestation for lumber extraction, agriculture, and charcoal production, as well as hunting have reduced the habitat and caused declines in the population of GLT [29, 39]. To confront this, The Golden Lion Tamarin Conservation Program (GLTCP) initiated a long-term conservation effort in 1983, including field research, management of the tamarins' population and its habitat, a captive-breeding program, creation and management of protected areas, environmental education, local capacity building and influencing public policies and many other actions. As a consequence, the golden lion tamarins became internationally recognized as a flagship species and a symbol of Atlantic Forest conservation.
The effort involved in translocating wild and threatened groups to a new area, forming a new population, was an important action aimed at preserving wild population of the GLT. This resulted in preservation of another area of forest, now converted into a Federal Biological Reserve (Fig. 6). Currently, the União Biological Reserve population represents 15% of the total wild population of the GLT.
The GLTCP is a model of landscape-scale integrated management, with an impact on the region. Now the local community is giving increased value to its natural environment, rescuing their culture and history and increasing their participation in conservation and ecotourism activities (Fig.7). These efforts resulted in the change of threat category for the GLT. The 2003 IUCN Red List re-classified and upgraded the status of L. rosalia from critically endangered to endangered [72].

Fig. 7. An effigy of a golden lion tamarin as a public telephone booth in a municipality in the species' area of distribution. © AMLD
A few years ago, the Brazilian Institute for the Environment team of the União Biological Reserve (UBR) started an environmental education program aimed at neighboring communities. Most of people in these communities had lived or worked in the area when the land belonged to the Federal Railroad Company. The change from an industrial activity to a conservationist activity today needs to be better explained to the neighboring populations, so that they can become an integral component of community-oriented conservation efforts. Approximately 3,000 people live in the neighborhood of the UBR. The environmental-conservation oriented programs are aimed at raising conservation awareness among the general public, students, and teachers. Our approach is to transmit the concept that the UBR can be an important instrument in learning about the environment (Fig. 8).

Fig. 8. Environmental education activities in local communities. Left © P. Procópio-de-Oliveira; right © AMLD)
Although w
e do not have data on patterns of seed dispersal for other frugivorous species in UBR before translocation of L. rosalia (which would allow us to compare these with patterns of seed deposition nowadays), we believe the tamarins are contributing importantly to seed dispersal and seedling establishment. The tamarins ingest a great number of seeds of many species, they have short gut passage time and they tend to move away from the fruiting tree shortly after eating, traversing various distances and defecating seeds outside the parental seed shadow. Previous studies indicate that Leontopithecus rosalia disperse the seeds of a large number of plant species and that the seeds remain viable for germination [28]. While our study showed that the tamarins spit the seeds of 21 species under the parent tree, data also indicated that they ingest and disperse the seeds of 76 fruit species. According to Fuentes [73], the recruitment of many plant species is limited more by the failure of seeds to arrive at appropriate places than by their failure to establish themselves in germination sites. The moustached tamarin (Saguinus mistax) and the saddle-back tamarin (S. fuscicollis) are reported to serve as dispersal agents for a variety of Amazon rain forest plants, exerting a direct influence on the regeneration and floristic heterogeneity of their area [10]. The black-handed tamarin (Saguinus midas niger) plays an important role in the regeneration of degraded and fragmented forest due to its preference for edge habitat [26]. The fact that the GLT travels across the habitats in the study area and that it also visits edges and degraded areas, suggests that seeds are transported to diverse habitats where germination and establishment may occur.
Acknowledgements
The study was supported by FAPESP (99/10860-8 and 02/09293-6), USAID/WWF-Brasil (CSR186-2000), Cleveland Metroparks Zoo/Scott Neotropical Fund and CI/Primate Action Fund. We are grateful to the Depto. de Ecologia/IB (USP) and Associação Mico-Leão-Dourado (AMLD) for institutional support. We also thank the International Committee for the Conservation and Management of Lion Tamarins (ICCM for Leontopithecus) and IBAMA for permission to work in the UBR. We thank Dr. Devra Kleiman for English corrections and suggestions, Dr. José Carlos Motta-Junior and Dr. Paulo Nogueira-Neto (Depto. de Ecologia-USP), and the Translocation Team: M.Cecília Kierulff, Sandro V. da Rocha, Susie Pinto, Nailton Azevedo, Hamilton Camargo-Filho, Mateus Carvalho, Walter Silva. We also thank four anonymous reviewers for helpful comments on earlier versions of this manuscript. Finally, we are grateful to all the people who collaborated on this work.
References
[1] Janzen, D.H. 1970. Herbivores and the number of trees species in tropical forests. American Naturalist 104: 501-527.
[2] Connell, J.H. 1971. On the role of natural enemies in preventing competitive exclusion in some marine animals and rain forest trees. In P.J. Dem Boer and G.R. Gradwell, (eds.), Dynamics of Populations pp. 298-312. Centre for Agricultural Publishing and Documentation, Wageningen.
[3] Howe, H.F. and J. Smallwood. 1982. Ecology of seed dispersal. Annual Review of Ecology and Systematics 13:201-228.
[4] Tutin, C.E.G., E.A. Williamson, M.E. Rogers and M. Fernandez M. 1991. A Case study of a plant-animal relationship Cola lizae and lowland gorillas in the Lopé Reserve, Gabon. Journal of Tropical Ecology 7: 181-199.
[5] Augspurger, C.K. 1984. Seedling survival of tropical tree species: interactions of dispersal distance, light-gaps, and pathogens. Ecology 65(6):1705-1712.
[6] Stevenson, P.R. 2007. A test of the escape and colonization hypotheses for zoochorous tree species in a Western Amazonian forest. Plant Ecology 190: 245-258.
[7] Reid, N. 1989. Dispersal of mistletoes by honeyeaters and flowerpeckers: components of seed dispersal quality. Ecology 70: 137-145.
[8] Schupp, E.W. 1988. Factors affecting post-dispersal seed survival in a tropical forest. Oecologia 76:525-530.
[9] Chapman, C.A. and D.A. Onderdonk. 1998. Forest Without Primates: Primate/Plant Codependency. American Journal of Primatology 45(1):127-141.
[10] Garber, P.A. 1986. The Ecology of Seed Dispersal in Two Species of Callitrichid Primates (Saguinus mystax and Saguinus fuscicollis). American Journal of Primatology 10: 155-170.
[11] Garber, P.A. and J.E. Lambert. 1998. Primate as seed dispersers: ecological processes and directions for future research. American Journal of Primatology 45(1): 3-8.
[12] Schupp, E.W. 1993. Quantity, quality and the effectiveness of seed dispersal. Vegetatio 107/108: 15-29.
[13] Chapman, C. 1989. Primate seed dispersal: the fate of dispersed seeds. Biotropica 21(2): 148-154.
[14] Traveset, A. 1998. Effect of seed passage through vertebrate frugivores' guts on germination: a review. Perspectives in Plant Ecology, Evolution and Systematic 1(2): 151-190.
[15] Nathan, R. and H.C. Muller-Landau. 2000. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends in Ecology and Evolution 15(7): 278-285.
[16] Knogge, C., E.W. Heymann and E.R.T. Herrera. 1998. Seed dispersal of Asplundia peruviana (Cyclanthaceae) by the primate Saguinus fuscicollis. Journal of Tropical Ecology 14: 99-102.
[17] Bullock, S.H. and R. Primack. 1977. Comparative experimental study of seed dispersal on animals. Ecology 58(3): 681-686.
[18] Zhang, S.Y and L.X. Wang. 1995. Comparison of tree fruit census methods in French Guiana. Journal of Tropical Ecology 11: 281-294.
[19] Stevenson, P.R. 2000. Seed dispersal by woolly monkeys (Lagothrix lagothricha) at Tinigua National park, Colombia: dispersal distance, germination rates, and dispersal quantity. American Journal of Primatology 50(4): 275-289.
[20] Lambert, J.E. 1998. Primate digestion: Interactions among anatomy, physiology, and feeding ecology. Evolutionary Anthropology 7: 8-20.
[21] McConkey, K.R. 2000. Primary seed shadow generated by gibbons in the rain forest of Barito Ulu, Central Borneo. American Journal of Primatology 52(1): 13-29.
[22] Stevenson, P.R., M.C. Castellanos, J.C. Pizarro and M. Garavito. 2002. Effects of seed dispersal by three ateline monkey species on seed germination at Tinigua National Park, Colombia. International Journal of Primatology 23 (6): 1187-1204.
[23] Chapman, C. 1995. Primate seed dispersal: Coevolution and conservation implications. Evolutionary Anthropology 4: 74-82.
[24] Knogge, C. and E.W. Heymann. 2003. Seed dispersal by sympatric Tamarins, Saguinus mystax and Saguinus fuscicollis: diversity and characteristics of plant species. Folia Primatologica 74: 33-47.
[25] Knogge, C., E.R.T. Herrera and E.W. Heymann. 2003. Effects of passage through tamarin guts on the germination potential of dispersed seeds. International Journal of Primatology 24(5): 1121-1128.
[26] Oliveira, A.C. and S.F. Ferrari. 2000. Seed dispersal by black-handed tamarins, Saguinus midas niger (Callitrichinae, Primates): implications for the regeneration of degraded forest habitats in eastern Amazonia. Journal of Tropical Ecology 16: 709-716.
[27] Passos, F.C. 1997. Seed dispersal by black lion tamarin, Leontopithecus chrysopygus (Primates, Callitrichidae), in southeastern Brazil. Mammalia 61(1): 109-111.
[28] Lapenta, M.J., P. Procópio-de-Oliveira, M.C.M. Kierulff and J.C. Motta-Junior. 2008. (In press). Frugivory and seed dispersal of golden lion tamarin (Leontopithecus rosalia (Linnaues 1766)) in a forest fragment, in the Atlantic forest, Brazil. Brazilian Journal of Biology 68(2).
[29] Kierulff, M.C.M. and P. Procópio-de-Oliveira. 1996. Re-assessing the status and conservation of the Golden Lion Tamarin (Leontopithecus rosalia) in the wild. Dodo, J. Jersey Wildlife Preservation Trust 32: 98-115.
[30] Kierulff, M.C.M. and P. Procópio-de-Oliveira. 1998. Reserva Biológica Fazenda União, Rio de Janeiro. Neotropical Primates 6(2): 51.
[31] Procópio-de-Oliveira, P.; M.C.M. Kierulff, M.J Lapenta, A.Martins, B. Beck. 2008. Técnicas de Manejo para a Conservação do Mico-Leão-Dourado. In Procópio de Oliveira, P.; C. Ruiz-Miranda and A.D. Grativol, (orgs.), Conservação do Mico-Leão-Dourado: enfrentando os desafios de uma paisagem fragmentada, pp. 118-135Editora UENF, RJ.
[32] Kleiman, D.G., R.J. Hoage and K.M. Green. 1988. The lion tamarins, genus Leontopithecus. In R.A. Mittermeier, A.B. Rylands, A.F. Coimbra-Filho and G.A.B. Fonseca, (eds.), Ecology and Behaviour of Neotropical Primates II, pp. 299-347. World Wildlife Fund, Washington, D.C.
[33] Procópio-de-Oliveira, P. 2002. Ecologia alimentar, dieta e área de uso de Micos-Leões-Dourados translocados e sua relação com a distribuição espacial e temporal de recursos alimentares na Reserva Biológica União-RJ. Tese de Doutorado. Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais.
[34] Kierulff, M.C.M. 2000. Ecology and behaviour of translocated groups of golden lion tamarin (Leontopithecus rosalia). PhD. Thesis, Cambridge University, Cambridge, UK.
[35] Ruiz-Miranda, C., A.D Grativol and P. Procópio-de-Oliveira. 2008. A espécie e sua situação na paisagem fragmentada. In P. Procópio-de-Oliveira, C. Ruiz-Miranda and A.D. Grativol (ogs.), Conservação do Mico-Leão-Dourado: enfrentando os desafios de uma paisagem fragmentada, pp. 6-13, Editora UENF, Campos dos Goytacazes, RJ.
[36] IUCN (World Conservation Union) (2007). 2007. IUCN Red List of Threatened Species. IUCN — World Conservation Union, Gland, Switzerland.
[37] Kierulff, M.C.M., B.E Raboy, P. Procópio-de-Oliveira, K. Miller, F.C. Passos and F. Prado. 2002. Behavioral ecology of lion tamarins. In D.G. Kleiman and A.B. Rylands (eds.), Lion Tamarins Biology and Conservation, pp. 157-187, Smithsonian Institution Press.
[38] Rylands, A.B., M.C.M. Kierulff and L.P.S. Pinto. 2002. Distribution and status of lion tamarins. In D.G. Kleiman and A.B. Rylands (eds.), Lion Tamarins Biology and Conservation, pp. 42-58. Smithsonian Institution Press.
[39] Coimbra-Filho, A. 1969. Mico-Leão, Leontideus rosalia (Linnaeus, 1766), situação atual da espécie no Brasil (Callitrichidae - Primates). Annais da Academia Brasileira de Ciência 41(suplemento): 29-52.
[40] Dietz, J.M., C.A. Peres and L. Pinder. 1997. Foraging ecology and use of space in wild golden lion tamarins (Leontopithecus rosalia). American Journal of Primatology 41: 289-305.
[41] Procópio-de-Oliveira, P., M.C.M. Kierulff and M.J. Lapenta. 2008. Dieta e área de uso de micos-leões-dourados na Reserva Biológica União, RJ. In P. Procópio-de-Oliveira, C. Ruiz-Miranda and A.D. Grativol (ogs.), Conservação do Mico-Leão-Dourado: enfrentando os desafios de uma paisagem fragmentada, pp. 40-57. Editora UENF, RJ.
[42] Lapenta, M.J., P. Procópio-de-Oliveira, M.C.M. Kierulff and J.C. Motta-Junior. 2003. Fruit exploitation by Golden Lion Tamarins (Leontopithecus rosalia) in the Uniao Biological Reserve, Rio das Ostas, RJ—Bazil. Mammalia 67(1): 41-46.
[43] Lapenta, M.J., P. Procópio-de-Oliveira and P. Nogueira-Neto. 2007. Daily activity period, home-range and sleeping sites of golden lion tamarins (Leontopithecus rosalia) translocated to the União Biological Reserve, RJ-Brazil. Mammalia 71(3): 131-137.
[44] Zar, J.H. 1984. Biostatistical Analysis. 2ed., Prentice-Hall, New Jersey.
[45] Forget, P.M and D. Sabatier. 1997. Dynamics of the seedling shadow of a frugivore-dispersed tree species in French Guiana. Journal of Tropical Ecology 13: 767-773.
[46] Dew, J.L and P. Wright. 1998. Frugivory and seed dispersal by four species of primates in Madagascar's eastern rain forest. Biotropica 30(3): 425-437.
[47] Wehncke, E.V, C.N. Valdez and C.A. Dominguez. 2004. Seed dispersal and defecation patterns of Cebus capucinus and Alouatta palliata: consequences for seed dispersal effectiveness. Journal of Tropical Ecology 20: 535-543.
[48] Howe, H.F. 1980. Monkey dispersal and waste of a neotropical fruit. Ecology 61(4): 944-959.
[49] Julliot, C. 1997. Impact of seed dispersal by red howler monkeys Alouatta seniculus on the seedling population in the understory of tropical rain forest. Journal of Ecology 85: 431-440.
[50] Serio-Silva, J.C. and V. Rico-Gray. 2002. Interacting effects of forest fragmentation and howler monkey foraging on germination and dispersal of fig seeds. Oryx 36(3): 266-271.
[51] Martins, M.M. 2006. Comparative seed dispersal effectiveness of sympatric Alouatta guariba and Brachyteles arachnoides in Southeastern Brazil. Biotropica 38(1): 57-63.
[52] Russo, S.E. 2005. Linking seed fate to natural despersal patterns: factors affecting predation and scatter-hoarding of Virola calophylla seeds in Peru. Journal of Tropical Ecology 21: 243-253.
[53] Ponce-Santizo, G., E. Andresen, E. Cano and A.D. Cuarón. 2006. Dispersión Primaria de Semillas por Primates y Dispersión Secundaria por Escarabajos Coprófagos em Tikal, Guatemala. Biotropica 38(3): 390-397.
[54] Russo, S.E, C.K. Augspurger. 2004. Aggregated seed dispersal by spider monkeys limits recruitment to clumped patterns in Virola calophylla. Ecology Letters 7: 1058-1067.
[55] Feer, F. 1999. Effects of dung beetles (Scarabaeidae) on seeds dispersed by howler monkeys (Alouatta seniculus) in the French Guianan rain forest. Journal of Tropical Ecology 15: 129-142.
[56] Voysey, B.C., K.E. McDonald, M.E. Rogers, C.E.G. Tutin and R.J. Parnell. 1999. Gorillas and seed dispersal in the Lopé Reserve, Gabon. I: Gorilla acquisition by trees. Journal of Tropical Ecology 15: 23-38.
[57] Rogers, M.E., B.C. Voysey, K.E. McDonald, R.J. Parnell and C.E.G. Tutin. 1998. Lowland gorillas and seed dispersal: The Importance of Nest Sites. American Journal of Primatology 45(1): 45-68.
[58] Chapman, C. and L.J. Chapman. 1996. Frugivory and the fate of dispersed and non-dispersed seeds of six African tree species. Journal of Tropical Ecology 12: 491-504.
[59] Estrada, A. and R. Coates-Estrada. 1991. Howler monkeys (Alouatta palliata), dung beetles (Scarabaeidae) and seed dispersal: ecological interactions in the tropical rain forest of Los Tuxtlas, Mexico. Journal of Tropical Ecology 7:459-474.
[60] Vulinec, K., J.E. Lambert and D.J. Mellow. 2006. Primate and dung beetle communities in secondary growth Rain Forest: implications for conservation of seed dispersal systems. International Journal of Primatology 27(3):855-879.
[61] Pizo, M.A. and P.S. Oliveira. 2000. The use of fruits and seeds by ants in the Atlantic Forest of Southeast Brazil. Biotropica. 32(4b):851-861.
[62] Forget, P.M. and T. Milleron. 1991. Evidence for secondary seed dispersal by rodents in Panama. Oecologia 87:596-599.
[63] Castilla, A.M. 2000. Does passage through the lizard Podarcis lilfordi's guts affect germination performance in the plant Withania frutescens? Acta Oecologica 21(2): 119-124.
[64] Estrada, A. and R. Coates-Estrada.1984. Fruit eating and seed dispersal by Howling Monkeys (Alouatta palliata) in the tropical rain forest of Los Tuxtlas, Mexico. American Journal of Primatology 6: 77-91.
[65] Milton, K., P.J. Van Soest and J.B. Robertson. 1980. Digestive efficiencies of wild howler Monkeys. Physiological Zoology 43(4): 402-409.
[66] Milton, K. 1981. Food choice and digestive strategies of two sympatric primates species. American Naturalist 117(4): 496-505.
[67] Remis, M.J. 1997. Western lowland gorillas (Gorilla gorilla gorilla) as seasonal frugivores: use of variable resources. American Journal of Primatology 43: 87-109.
[68] Gathua, M. 2000. The effects of primates and squirrels on seed survival of a canopy tree, Afzelia quanzensis, in Arabuko-Sokoke Forest, Kenya. Biotropica 32(1): 127-132.
[69] Trakhtenbrot, A., R. Nathan, G. Perry and D.M. Richardson. 2005. The importance of long-distance dispersal in biodiversity conservation. Diversity and Distribution 11: 173-181.
[70] Fleming, T.H. and C.F. Williams. 1990. Phenology, seed dispersal, and recruitment in Cecropia peltata (Moraceae) in Costa Rican tropical dry forest. Journal of Tropical Ecology 6: 163-178.
[71] Jordano, P. and J.A. Godoy. 2002. Frugivore-generated seed shadows: a landscape view of demographic and genetic effects. In Levey, D.J., W. R. Silva and M. Galetti (eds.), Seed dispersal and frugivory : ecology, evolution, and conservation, pp. 305-322. CABI Pub., New York.
[72] IUCN (World Conservation Union) (2003). 2003. IUCN Red List of Threatened Species. IUCN — World Conservation Union, Gland, Switzerland.
[73] Fuentes, M. 2000. Frugivory, seed dispersal and plant community ecology. Trends in Ecology and Evolution 15(12): 487-488.
|










