|
Tropical dry evergreen forests of peninsular India: ecology and conservation significance
N. Parthasarathy1*, M. Arthur Selwyn1 and M. Udayakumar1
1Department of Ecology and Environmental Sciences, Pondicherry University, Puducherry — 605 014, India. *Email: [email protected]
Abstract
Tropical dry evergreen forests (TDEFs) occur as patches along the Coromandel coast of peninsular India. Investigations on plant biodiversity, bioresource values, and conservation status of 75 TDEF sites were carried out. A total of 149 woody plant species representing 102 trees, 47 lianas, and three native herbs were enumerated. Across 75 sites studied, species richness of woody plants ranged from 10 to 69 species. Physiognomically, evergreen species dominated the forest. Forest growth determined as girth increment ranged from 0.37 to 1.08 cm yr-1 for trees and 0.39 to 0.41 cm yr-1 for lianas. At the community level, seasonal flowering with unimodal dry season peak and year-round, bimodal fruiting pattern prevailed. A strong association between the qualitative reproductive traits and pollination and dispersal spectrum among the TDEF species has been demonstrated. In bioresource assessment, 150 medicinal plant species, used for treating more than 52 ailments, were documented. Site disturbance scores were obtained by assessing the various site disturbances such as site encroachment, resource extraction, grazing, fragmentation, weed invasion, etc. Conservation significance of the TDEF sites is emphasized in the light of restricted geographical distribution, moderate level of plant species diversity, representation of the unique forest type, high productivity, and bioresource potential. Restoring the disturbed sites with characteristic TDEF species, and revitalizing the cultural traditions associated with sacred groves by promoting awareness of the ecological and bioresource values of TDEFs, are recommended.
Key words: Tropical dry evergreen forest, biodiversity, functional ecology, bioresource value, conservation significance
|
Received: 30 January 2008, Accepted: 20 March, 2008, Published: 9 June, 2008
Copyright: © 2008 Parthasarathy et al.This is an open access paper. We use the Creative Commons Attribution 3.0 license http://creativecommons.org/licenses/by/3.0/us/ - The license permits any user to download, print out, extract, archive, and distribute the article, so long as appropriate credit is given to the authors and source of the work. The license ensures that the published article will be as widely available as possible and that your article can be included in any scientific archive. Open Access authors retain the copyrights of their papers. Open access is a property of individual works, not necessarily journals or publishers
Cite this paper as: Parthasarathy N., Arthur Selwyn M. and Udayakumar M. 2008.
Tropical dry evergreen forests of peninsular India: ecology and conservation significance. Tropical Conservation Science Vol.1(2):89-110. Available online: tropicalconservationscience.org
Introduction
In the tropics, changes in the quantity and distribution of rainfall along with temperature and the length of the dry season gradually alter the vegetation formation [1]. The pronounced seasonality in rainfall distribution with several months of drought result in seasonally dry forests in tropical regions [2]. As dry forests have a broad climatic range, transitional forest ecosystems like grasslands, savannas, scrub, and thorn woodlands are often considered during the vegetation assessments [3]. Many times, these dry forests in the varying climatic regimes differ in their forest structure and physiognomy [2, 4]. The prevailing tropical dissymmetric climate regime on the Coromandel coast of southern peninsular India supports a unique type of vegetation named tropical dry evergreen forest (TDEF) [5]. The Coromandel coastal plains extend about 80-100 km inland [6].
The tropical dry evergreen forest (TDEF) type, first described as a low forest of 9 to 12 m high, forms, however, a complete canopy comprising small, coriaceous-leaved evergreen trees of short boles and spreading crowns with some deciduous emergents, without marked differentiation of canopy layers [5]. Floristically, it is distinguished by a fair representation of characteristic and preferential species, exclusively or mostly confined to this vegetation type [5, 7]. The tropical dry evergreen forests on the Coromandel coast of India, which occur as patches, are short-statured, largely three-layered, tree-dominated evergreen forests with a sparse and patchy ground flora [8].
Distribution of tropical dry evergreen forest
Dry evergreen forests have also been reported elsewhere in the tropics as summarized in Table 1, along with aspects researched therein. The information provided in Table 1 is a result of a systematic review that has been made possible through extensive survey of literatures that report the occurrence of dry evergreen forests, either as a vegetation formation or as a forest type. There are no unified features for this rare and unique forest type and it has been chosen based on local climatic, biotic and edaphic factors, which influence the forest's physiognomy, stand structure, species composition, and dynamics.
Table 1. Distribution of tropical dry evergreen forests in the tropics and aspects studied therein. (* Cl-Climate; Sl-soil; Veg-vegetation structure; FC-floristic composition; Dyn-dynamics; Phy-physiology; N.Cy-nutrient cycling; Repr-reproductive ecology; FU-forest utilization; T&D-threats and disturbance; Con-conservation)
|
Location |
Aspects studied* |
Reference |
|
Cl |
Sl |
Veg |
FC |
Dyn |
Phy |
N.Cy |
Repr |
FU |
T&D |
Con |
|
Tropical America
|
|
Antigua |
v |
v |
v |
v |
|
|
|
|
|
|
|
16 |
|
Bahamas |
v |
v |
v |
v |
|
|
|
|
|
v |
|
10-13 |
|
British Guiana
|
v |
v |
v |
v |
|
|
|
|
|
|
|
15-16 |
|
Jamaica |
v |
v |
v |
v |
|
|
|
|
|
|
|
17-19 |
|
Trinidad |
v |
v |
v |
v |
|
|
|
|
|
|
|
20 |
|
Tobago |
v |
v |
v |
v |
|
|
|
|
|
|
|
21 |
|
Africa
|
|
Ethiopian highlands |
v |
v |
v |
v |
|
|
|
|
v |
v |
v |
22 |
|
Tanzania |
v |
v |
v |
v |
|
|
v |
|
|
|
v |
23-24 |
|
Zambia |
v |
v |
v |
v |
|
|
|
|
|
v |
|
25-26 |
|
Asia
|
|
Thailand |
v |
v |
v |
v |
v |
v |
v |
|
v |
v |
v |
27-31 |
|
Sri Lanka |
v |
v |
v |
v |
v |
v |
v |
|
v |
v |
v |
32-34 |
|
India |
v |
v |
v |
v |
v |
|
v |
v |
v |
v |
v |
5, 35-46, 49-52, 54-58, 64 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beard (1955) [9] recognized six dry evergreen formations in tropical America, which are formed due to strong winds and/or excessively freely draining soil, whereas the rainfall regime there is not of dissymmetric type. In tropical America, TDEF occurs in the North Andros islands of the Bahamas as a "coppice community," which is a dense, close-canopied, broad-leaved evergreen forest [10-13], and in British Guiana [14, 15], Antigua [16], Jamaica [17-19], Trinidad [20], and Tobago [21]. In Africa, TDEF is reported as montane evergreen scrubland vegetation in multi-storied form in the highlands of Ethiopia [22], and in Tanzania and north-eastern Zambia as scattered patches of closed canopy of evergreen shrubs of 15-25 m tall (locally, known as "Matechi") [23-26].
Dry evergreen forests as a closed-canopy evergreen forest type, with 25-30 m of mean canopy height, are widespread in the regions of Thailand that receive not more than 1,200 mm mean annual rainfall, with 4-6 dry months [27-31]. In Sri Lanka, dry evergreen forest is typical and a dominant vegetation type in the dry zone regions in the northern and eastern plains, which cover 80 percent of the island area [32-34]. In India, this vegetation is confined to the Coromandel (east) coast region [5, 8, 35-42]. Some patches of dry evergreen forests have also been recorded in the Sirumalai hills [43], Kolli hills [44], Shervarayan hills [45] and Chitteri hills [46] of southern Eastern Ghats. However, the climate and characteristic species of hill dry evergreen type are not the same as that of the coastal region.
In reality, most of the Indian TDEFs, with the exception of two large areas, namely the Kurumbaram section of the Marakanam Reserve Forest and the Point Calimere Wildlife Sanctuary, occur as patches of forest dotted along the Coromandel coast, and invariably protected as "sacred groves" based on the religious belief of the local people. This unique dry evergreen forest is relatively under-studied on aspects of structural and functional ecology, as compared to the tropical wet evergreen forests. The aim of this paper is to provide a consolidated account on plant biodiversity, structure and functional ecology, and bioresource potential, particularly of medicinal plants, and to emphasize the conservation need and significance of TDEFs on the Coromandel coast of peninsular India.
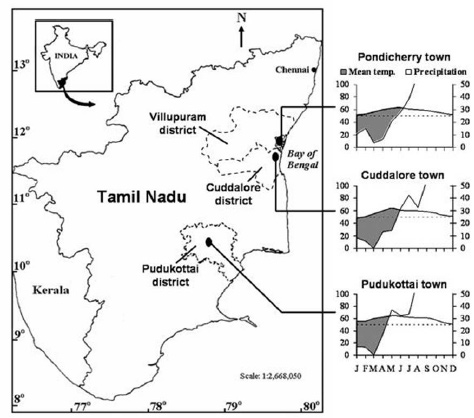
Fig.1. Map showing the districts, viz. Villupuram (5 sites), Cuddalore (28 sites), Pondicherry (5 sites) and Pudukottai (37 sites), wherein 75 TDEFs are located on the Coromandel coast of peninsular India and climate diagram for three nearest towns, which depict the tropical dissymmetric climate regime.
Methods
Study area
Investigations on plant biodiversity and bioresource potential of 75 TDEF sites, which are concentrated in the Pondicherry (11°56' N and 79°53' E), Villupuram (11°93' N and 79°48' E), Cuddalore (11°43' N and 79°49' E) and Pudukottai (10°23' N and 78°52' E) districts on the Coromandel coast of peninsular India, were carried out (Fig. 1). The areal extent of TDEF sites studied ranged from 0.5 ha to ~10 ha. The climate is tropical dissymmetric type with most rainfall received during the northeast monsoon (October-December) and very little and inconsistent rainfall in the southwest monsoon (June to September). The mean annual rainfall is 1,282, 1,079 and 1,033 mm in the nearest towns, namely Pondicherry, Cuddalore, and Pudukottai, respectively. The dry season lasts for six months (January to June), and receives less than 60 mm rainfall on monthly average. Mean annual maximum and minimum temperatures are 32.58°C and 24.51°C in Pondicherry, 22.75°C and 33.64°C in Cuddalore, and 33.4°C and 25.4°C in Pudukottai.
Data collection
Field data collection on species check listing and assessment of bioresource values in the 75 TDEF sites was conducted in about 125 man days during July 2006-January 2008. The dataset on woody plant diversity (for trees equal or greater than 10 cm girth at breast height, (1.3 m height from ground level), and all lianas =1 cm diameter measured at 1.3 m from the base of the stem), dynamics, and functional ecology reviewed here is based on the systematic investigations carried out in a total of 12 one-ha permanent plots over a decade on the Coromandel coast of peninsular India. All plant species were identified and confirmed to species level using regional floras [47-48]. Voucher specimens were collected and deposited in the herbarium of the Department of Ecology, Pondicherry University.
Site disturbance scores were obtained by assessing various disturbances (on a 1-5 scale) which include site encroachment, distance from the human habitation, temple visitors' impact, cattle grazing, resource removal, width of approach road to temple, fragmentation, size of the temple, biological invasion, and frequency of peoples' visit to the temple (refer to Appendix 1 for more details). The summed disturbance score of each site was used for ranking the TDEF sites into three categories, viz relatively undisturbed (Fig. 2a&b), moderately disturbed (Fig. 2c&d) and highly disturbed (Fig. 2e&f), for evaluating the conservation significance. The sites with low ranks experience least disturbance, while high ranks reveal a high level of anthropogenic disturbance in the site.
Medicinal plant resource use and traditional knowledge related to plant species of TDEFs were collected through a field-tested improved questionnaire (Appendix 2) and personal interviews with folk healers in their vernacular language. There were 47 informants (40 males and 7 females), who are folk healers by profession and part of the local folk healers association.
Results and Discussion
Biodiversity
In a total of 75 TDEF sites on the Coromandel coast of peninsular India, 149 woody species that belonged to 122 genera and 49 families were enumerated (Appendix 3). In addition to these, three important native herbaceous species occur there, which include the widely-distributed, colony-forming Sansevieria roxburghiana, fairly distributed Ecbolium viride, and the rare Amorphophallus sylvaticus. Dominant families in Indian TDEFs include Euphorbiaceae, Rubiaceae with 11 species each, followed by Capparaceae, Mimosaceae, Fabaceae and Moraceae with 8 species each, while Alangiaceae, Barringtoniaceae and Burseraceae are represented by single species. Across 75 sites studied, species richness of woody plants ranged from 10 (Azhiyanilai and Thakkiripatti) to 69 species (Puthupet), and sites with more than 50 species include Puthupet (69 species), Kuzhanthaikuppam (53), Shanmuganathapuram (53) and Oorani (52). The Morisita-Horn index for similarity of species composition between 75 TDEF sites (1.0 indicates total similarity) varied from 0 to 0.8 and only 10% of pairs had = 0.5, indicating the greater heterogeneity in the composition of species.

Fig. 2. Forest and interior stand view of relatively undisturbed (a&b), moderately disturbed (c&d) TDEF sites, landscape of TDEF with goat herding (e) and part of site converted to Acacia monoculture(f). a. Site OR-View of TDEF vegetation; b. RP- Chloroxylon-Pterospermum dominated stand; c. IT- Forest and sacred grove in entrance; d. MN-Inner stand view; e. KT-Herding goats inside the forest; f. OME-Converted to Acacia leucophloea (Mimosaceae) monoculture.
Among life forms, trees were dominant (102 species) representing 68% of the total species, while lianas formed 32% (47 species). Tree species richness at individual site ranged from 9 in Mettupatti to 36 species in Shanmuganathapuram. Mean species richness of lianas at each site was 30%. Maximum number of lianas was recorded at Puthupet (33 species), whereas lianas were virtually absent at Thakkiripatti. Some unique TDEF species such as Pterospermum xylocarpum, Millusa montana, Polyalthia suberosa, and Alangium salvifolium among trees, and Olax scandens, Capparis rotundifolia, Pachygone ovata, and Mearua oblongifolia among lianas occurred only in a few sites.
Earlier quantitative ecological inventory of plant biodiversity in 12 1-ha TDEF permanent plots [39-41, 49-51] has resulted in 86 tree species with a range of 19 to 35 species (Fig.3a-f). A ubiquitous tree, Memecylon umbellatum, was the most dominant species, accounting for 32% of tree density, followed by Tricalysia sphaerocarpa (10.5%) and Pterospermum canescens (9.7%) in the tropical dry evergreen forests. A total of 44 liana species was inventoried with a range of 21-29 species ha-1 in the eight 1-ha plots. Among the lianas, Combretum albidum (19.2%), Strychnos minor (14%), and Reissantia indica (6.5%) were predominant species.
Although the 75 sites studied belong to the same TDEF type and are grossly homogeneous, they differ in forest stature; sites occurring on sandy soil with alluvium deposits are comparatively tall-statured (mean ht ~12 m; eg. TM, OR, AP, etc.) and those on red ferralitic hard compact soil are short- to medium-statured (mean ht <8 m; eg. TK, KP, MK, etc.). There is a wide variation in species composition of tree and liana species across TDEF sites, and interestingly each site is dominated by a different set of tree and liana species, which can be designated as "series," adding to the uniqueness of the studied TDEF sites (e.g., Manilkara hexandra in SV and Memecyclon umbellatum-Tricalysia sphaerocarpa-Diospyros ebenum in KK among trees; Strychnos minor-Jasminum angustifolium in PP and Reissantia indica-Strychnos minor-Combretum albidum in OR among lianas).
Forest structure, growth, and dynamics
Out of 149 species, 75 are evergreen (50%), followed by deciduous (45 species, 30%) and brevi-deciduous species [species with brief deciduous period followed by synchronous leaf-flushing, e.g., Pterospermum canescens] (29 species; 20%). Among the 26 most common species, which occurred in more than 30 sites, 54% were evergreen, 31% deciduous, and 15% brevi-deciduous. Similar results were reported in the study conducted in 43 TDEF sacred grove sites that contained 48% to 85% evergreen species [52]. Among the three physiognomic groups, evergreenness was prominent among trees (49%) and lianas (53%). The naturally evolved assemblage of evergreen species in dry evergreen vegetation type may be related to leaching of nutrients from leaves and year-round leaf fall, which is characteristic of evergreen species that establish a more closed nutrient cycle in the forest [53]. Litter production quantified in two TDEF sites, namely Kuzhanthaikuppam (KK) and Oorani (OR), revealed a year-round litterfall with unimodal summer peak [54]. Leaf litter production amounted to 9.6 and 9 t ha-1 yr-1 at KK and OR, respectively, while the standing crop of total forest floor litter was 4.11 t ha-1 at KK and 4.86 t ha-1 at OR.
Plant population changes have been studied in seven TDEFs by measuring the tree growth, recruitment, and mortality rate over years [55-56]. Forest growth determined as girth increment in TDEF sites ranged from 0.37 to 1.08 cm yr-1 for trees and 0.39 to 0.41 cm yr-1 for lianas over a three-year period (2003-2006). The tree recruitment rate ranged from 0.7 to 2.3% yr-1, while the mortality rate ranged from 1 to 2.2% yr-1 in five TDEF sites studied over three years (2003-2006). More small trees (10-30 cm gbh) recruitment in the forest has been attributed to selective logging of trees of highest girth class (>150 cm gbh) for temple construction that allowed more canopy gaps and sunlight. Above ground biomass of 10 TDEF sites was estimated between 39.69 and 170.02 Mg ha-1 with a mean of 102.15 Mg ha-1 [57]
Reproductive ecology
Analysis of qualitative reproductive traits of TDEF species [58] revealed that many species had rotate-type, white-colored, scented flowers with nectar and pollen as rewards. Drupe and berry were the common fruit types and were found in black and red color, respectively. A strong association between the qualitative reproductive traits and pollination and dispersal spectrum among the TDEF species has been demonstrated [58]. Phenological observations on TDEF species revealed a seasonal and unimodal flowering pattern with dry season peak at the community level. A similar pattern of dry season flowering peak is also reported in other tropical seasonal dry forests [59-63]. Many species exhibited annual flowering except a few species such as Garcinia spicata, Reissantia indica, Dodonaea angustifolia, etc., which exhibited a sub-annual pattern. The deciduous species (e.g., Lannea coromandelica, Butea monosperma) displayed flowering and leaf shedding in dry summer. Species that flower during the high temperature and less rainfall attract diverse insects, while bee pollination was the prevalent mode of pollination system (68% of species) in the TDEF.
A bimodal fruiting pattern with a major peak in the dry season and a minor one in the early wet season was exhibited at the community level [64]. There was year-round fruit production without a clear seasonality, but fruiting patterns at species level showed pronounced seasonality, which is in conformity with other seasonal forests [62, 65-69]. The patterns of unimodal flowering and year-round fruiting pattern are common to seasonal dry tropical forests, and these patterns have evolved according to local climatic factors (temperature, rainfall, number of dry months) along with ecological factors like availability of pollinators and dispersers. Most trees in our TDEFs flower (63%) and fruit (50%) during the dry period, whereas lianas had major flowering (77%) and fruiting (57%) activity in the late wet to dry season of the year. Many species are dispersed by animals, and had fruiting peak during the late dry season, which enables seed germination and rapid seedling establishment at the onset of the rainy season. The community-level fruit production in TDEF sites averaged 757 kg-1ha-1yr-1 [70].

Fig. 3. Some characteristic tree and liana species of TDEFs a. Memecylon umbellatum (Melastomataceae)-predominant tree of TDEFs; b. Pterospermum canescens (Sterculiaceae) with woody capsule-common, lofty tree endemic to Coromandel coast TDEFs; c. Aglaia elaegnoidea (Meliaceae) - vertebrate-dispersed berries; d. Eugenia bracteata (Myrtaceae)- flowering twig; e. Hugonia mystax (Linaceae)-hook climber; f. Capparis brevispina (Capparaceae)-common thorny scrambler.
Our understanding of species biology, particularly reproductive ecology, is still in an infant stage, and future directions for promising research include: (a) the environmental cues, which influence the phenological pattern; (b) the reproductive biology of important species (trees: Pterospermum xylocarpum, Casearia elliptica, Aglaia elaegnoidea; lianas: Tiliacora acuminata and Strychnos minor; herbs: Sansevieria roxburghiana and Amorphophallus sylvaticus) and also of dioecious tree species to assess the minimum viable population; (c) the extent of specialization in plant-pollinator interactions; (d) the level of inbreeding within and among species; (e) the impact of habitat fragmentation on pollination and fruit dispersal; and (f) genetic diversity analysis of polymorphic species such as Memecylon umbellatum, Pterospermum xylocarpum, etc.
Implications for Conservation
Bioresource value
A total of 150 plant species that belonged to 57 families are reported to have medicinal value. They include 41 trees, 18 lianas, 14 shrubs, 10 herbaceous climbers, and 66 herbs. Andrographis paniculata, Phyllanthus amarus, Gymnema sylvestre, Solanum nigrum, and S. trilobatum are commonly used. A few characteristic/important medicinal species are featured in Figure 4. The proportion of plant species used for medicinal purpose classed by plant parts include leaves (41%), fruits and seeds (14%), bark (12%), root (8%), latex (7%), whole plant (6%), and flower and bulbs (1%). Traditional healers use these plants for curing more than 52 ailments, mainly poisonous bites (including snake, scorpion, dog, rat, beetle, bug, etc.), sexual diseases (including gonorrhea, syphilis. etc.), jaundice, rheumatism, skin diseases, ulcers, dysentery, diabetes, and common cold and fever.

Fig. 4. Selected medicinal plants from TDEFs. a. Sansevieria roxburghiana (Agavaceae)-endemic herb, medicinal & silky-fiber b. Strychnos nux-vomica (Loganiaceae)-seeds medicinal; c. Calophyllum inophyllum (Clusiaceae)- seed oil medicinal; d. Night blooming, fragrant-Tarenna asiatica (Rubiaceae); e. Strychnos minor (Loganiaceae)-Hook climber with foetid flowers; f. Cassia auriculata (Caesalpiniaceae)-leaves and flowers medicinal.
The bioresource potential, especially the medicinal importance of TDEF species, deserves detailed documentation in the additional unstudied sites. Further researches for bioresource augmentation and full utilization include: (a) developing propagation and nursery techniques for large-scale multiplication of multi-beneficial species and species of high medicinal importance such as Sansevieria roxburghiana (used for ear diseases and cough, and yielding silky fiber, face cream from leaf mucilage, sand binder, and a hedge plant), Amorphophallus sylvaticus (for piles), etc.; (b) phyto-chemical screening and bioprospecting of important species such as Memecylon umbellatum, Cassytha filiformis, Cissus vitiginea, Sarcostemma acidum, Atalantia monophylla, and Jasminum angustifolium.
Conservation significance
Overall disturbance scores of the 75 studied TDEF sites ranged from the lowest score of 13 for Suranviduthi to a maximum of 40 in Avudayarkoil and Azhagarkoil. Of the total 75 TDEF sites, 19 are relatively undisturbed (score range 13-20), but a moderate level of disturbance (score 21-30) is operative in 42 sites, whereas disturbance is severe (31-40) in 14 sites (Table 2). The declining trend in mean species richness was observed from relatively undisturbed to highly disturbed sites and a significant negative correlation (r = -0.534; P<001) existed when species richness was plotted against site disturbance scores (Fig. 5). In a linear regression analysis, when various site disturbance scores were regressed with species richness, site disturbances such as resource removal, frequency of peoples' visit to a temple, and forest areal extent had a greater influence on species richness of the site.
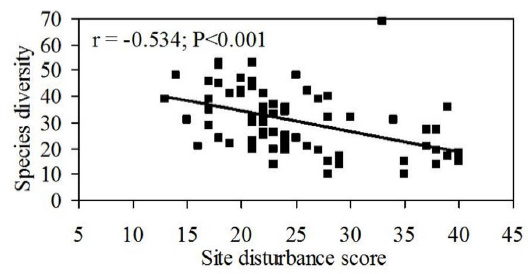
Fig. 5. Relation between site disturbances and species richness in 75 TDEF sites along the Coromandel coast of peninsular India
Having changed in land use patterns and shrunken acreage (0.5 to 3.5 ha), the highly disturbed TDEF sites are largely converted to monoculture plantations (Table 2). There are sites with least disturbance scores harboring the best natural dry evergreen vegetation with high diversity of plants of medicinal and cultural importance, and enhancing landscape heterogeneity as well as protecting the microclimates. Moderately disturbed sites are currently exposed to high level of threats, primarily from human intervention. Major issues like consistently increasing human habitation surrounding the forest area, poverty and illiteracy among large sections of the population, continuous areal shrinkage, over-exploitation, site degradation and land conversion are to be considered while assessing the conservation significance of each TDEF site.
Table 2. Conservation significance of TDEF sites ranked into three categories based on site disturbance scores, and illustrative site examples with their characteristic features.
|
Site disturbance |
Area (ha) |
Mean species richness |
No. of sites |
Illustrative site examples with characteristic features |
|
Relatively undisturbed (= 20) |
0.8-10 |
39.10 ±12.2 |
19 |
Araiyapatti - mono-dominant forest of Strychnos nux-vomica
Suranviduthi - old growth mono-dominant forest of Manilkara hexandra
Thirumanikuzhi — Tricalysia sphaerocarpa & Lepisanthes tetraphylla dominated
Shanmuganathapuram — mono-dominant forest of Memecylon umbellatum
Mavadipalayam — mono-dominant forest of Garcinia spicata
|
|
Moderately disturbed (21-30) |
1-8 |
29.33 ±12.2 |
42 |
Arasarkulam - old growth, fragmented forest; dominants — M. hexandra & Sapium insigne
Karukkai — culturally valued - temple; dominants - Atalantia monophylla & Pyrenacantha volubilis
Puthupet — high visitation, forest clearing in places for road & building construction
Keeranoor — more openness, dominant - Albizia amara
Kothattai — unique landscape in undulating one portion, old growth forest on sandy soil
|
|
Highly disturbed (31-40) |
0.5-3.5 |
20.38 ±7.6 |
14 |
Kadapakkam — high level of resource extraction
Azhiyanilai — converted native Acacia leucophloea plantation
Keezhoor — forest converted to tank
Embalam — converted to Khaya senegalensis plantation
Avudayarkoil — converted into Eucalyptus plantation
|
|
|
|
|
|
|
Fortunately, the sacred grove status of TDEF sites largely helped to preserve the biodiversity along with the cultural values and religious taboos. People conserve the forests in undisturbed sites through a strict code of conduct on religious beliefs for several generations without any legal administration and clearly defined management policy and make only minimal resource extraction. Cultural transformations, eroding cultural values, and the changing world view of nature, especially among younger generations, has made this traditional forest management worse [71] in many of the moderately and much disturbed sites.
In conclusion, the conservation of TDEF sites is important considering the restricted geographical distribution and representation of the unique and under-studied TDEF type, the extant level of biodiversity, and bioresource potential including medicinal plants and the socio-economic and ecological values of these systems. We recommend the following as long-term conservation strategies to preserve these sites: (a) promote awareness of biodiversity and bioresource values and cultural traditions associated with the sacred groves to people living around the TDEF sites—people who are also dependent on the forests and their resources and stand to benefit from conserving the sites that still remain relatively undisturbed; (b) restore moderately disturbed sites with characteristic TDEF species, involving the local communities in restoration programs and also in nurturing the planted saplings; (c) immediately protect and conserve much-disturbed sites by providing legal status to the forests and developing forest management systems involving the local community.
Acknowledgements
The authors thank two anonymous reviewers for their constructive suggestions, which have considerably improved this manuscript. NP and MU thank INSA for supporting medicinal plant study through a project on "History of Science" (INSA No.HS/RC/904/2006).
References
[1] Murphy, P. G., and Lugo A. E. 1986. Ecology of dry forest. Annual Review of Ecology and Systematics 17: 67-88.
[2] Mooney, H. A., Bullock S. H., and Medina E. 1995. Introduction. In: S. H. Bullock, H. A. Mooney and E. Medina (eds.), Seasonally dry tropical forests, pp.1-8. Cambridge University Press, Cambridge.
[3] Miles, L., Newton A. C., DeFries R. S., Ravilious C., May I., Blyth S., Kapos V., and Gordon J. E. 2006. A global overview of the conservation status of tropical dry forests. Journal of Biogeography 33: 491—505.
[4] Blasco, F., Whitmore T. C. and Gers C. 2000. A framework for the worldwide comparison of tropical woody vegetation types. Biological Conservation, 95: 175-189.
[5] Champion, H. G., and Seth S. K. 1968. Revised survey of the forest types of India. Manager of Publications, New Delhi.
[6] Mani, M. S. 1974. (Ed.) Ecology and biogeography in India. Dr. W. Junk B.V Publishers, The Haque, The Netherlands.
[7] Meher-Homji, V. M. 1974. On the origin of tropical dry evergreen forest of south India. International Journal of Ecology and Environmental Science 1: 19-39.
[8] Venkateswaran, R., and Parthasarathy N. 2005. Tree population changes in a tropical dry evergreen forest of south India over a decade (1992-2002). Biodiversity and Conservation 14: 1335-1344.
[9] Beard, J. S. 1955. The classification of tropical American vegetation types. Ecology 36: 89-100.
[10] Correll, D. S., and Correll H. B. 1982. Flora of the Bahama archipelago, J. Cramer, Vaduz, Liechtenstein.
[11] Smith, I. K. 1991. Dry evergreen forest (coppice) communities of North Andros Island, Bahamas. M.En. Thesis, Miami University, Oxford, OH.
[12] Smith, I. K., and Vankat J. L. 1992. Dry evergreen forest (coppice) communities of North Andros Island, Bahamas. Bulletin of the Torrey Botanical Club 119: 181-191.
[13] Nickrent, D. L., Eshbaugh W. H., and Wilson T. K. 1988. The vascular flora of Andros Island, Bahamas. Kendall/Hunt, Dubuque, IA.
[14] Fanshawe, D. B. 1952. The vegetation of British Guiana. A preliminary review. Imperial Forest Institute Paper No. 29, Oxford.
[15] Cornelissen, J. H. C., and Steege T. H. 1989. Distribution and ecology of epiphytic bryophyte and lichens in dry evergreen forest of Guyana. Journal of Tropical Ecology 5: 131-150.
[16] Loveless, A. R. 1960. The vegetation of Antigua, West Indies. Journal of Ecology 48: 495-527.
[17] Asprey, G. F., and Loveless A. R. 1958. The dry evergreen formation of Jamaica. II. The raised coral beaches of the north coast. Journal of Ecology 46: 547-570.
[18] Kelly, D. L., Tanner E. V. J., Kapos V., Dickinson T. A., Goodfriend G. A., and Fairbairn P. 1988. Jamaican limestone forests: floristics, structure and environment of three examples along a rainfall gradient. Journal of Tropical Ecology 4: 121-156.
[19] Loveless, A. R., and Asprey G. F. 1957. The dry evergreen formations of Jamaica. I. The limestone hills of the south coast. Journal of Ecology 45: 799-822.
[20] Beard, J. S. 1946. The natural vegetation of Trinidad. Oxford Forestry Memoir No. 20.
[21] Beard, J. S. 1944. The natural vegetation of Tobago. Ecological Monograph 14: 135-163.
[22] Woldu, Z. 1999. Forests in the vegetation types of Ethiopia and their status in the geographical context. In: S. Edwards, Demissie, Bekele and G. Haase (eds.), Forest Genetic Resource Conservation: Principles, Strategies and Actions; Proceedings of The National Forest Genetic Resources Conservation Strategy Development Workshop, 21-22 June 1999; Institute of Biodiversity Conservation and Research (IBCR) and the German Technical Co-operation (GTZ); Addis Ababa, Ethiopia.
[23] Kielland-Lund, J. 1982. Trees and shrubs in four forest and woodland communities near Morogoro. Division of Forestry Record, University of Dar-es-Salaam, Morogoro, Tanzania.
[24] Högberg, P. 1982. Mycorrhizal associations in some woodland and forest trees and shrubs in Tanzania. New Phytologist 92: 407-415.
[25] Trapnell, C. G. 1959. Ecological results of woodland burning experiments in Northern Rhodesia. Journal of Ecology 47:129-168.
[26] Lawton, R. M. 1978. A study of the dynamic ecology of Zambian vegetation. Journal of Ecology 66: 175-198.
[27] Ashton, P. S. 1990. Thailand: biodiversity center for the tropics of Indo-Burma. Journal of Science Society Thailand 16: 107-116.
[28] Bunyavejchewin, S. 1986. Ecological studies of tropical semi-evergreen rain forest of Thailand. Thai Forest Bulletin 14: 1-93.
[29] Bunyavejchewin, S. 1999. Structure and dynamics in seasonal dry evergreen forest in northeastern Thailand. Journal of Vegetation Science 10: 787-792.
[30] Santisuk, T. 1988. An account of the vegetation of Northern Thailand. Franz Steiner Verlag, Weisbaden.
[31] Pitman, J. I. 1996. Ecophysiology of tropical dry evergreen forest, Thailand: measured and modelled stomatal conductance of Hopea ferrea, a dominant canopy emergent. Journal of Applied Ecology 33: 1366-1378.
[32] Koelmeyer, K. O. 1957. Climatic classification and the distribution of vegetation in Ceylon. Ceylon Forester 3: 144-163.
[33] Dittus, W. P. J. 1985. The influence of cyclones on the dry evergreen forest of Sri Lanka. Biotropica 17: 1-14.
[34] Perrera, N. P. 1975. A physiognomic vegetation map of Sri Lanka (Ceylon). Journal of Biogeography 2: 185-203.
[35] Blasco, F., and Legris P. 1973. Dry evergreen forests of Point Calimere and Marakanam. Journal of Bombay Natural History Society 70: 279—294.
[36] Sprangers, J. T. C. M., and Balasubramaniam K. 1978. A phytosociological analysis of the tropical semi-evergreen forest of Marakkanam, south-eastern India. Tropical Ecology 19: 70-92.
[37] Parthasarathy, N., and Sethi P. 1997. Tree and liana species diversity and population structure in a tropical dry evergreen forest in south India. Tropical Ecology 38: 19-30.
[38] Visalakshi, N. 1992. Ecological studies in tropical dry evergreen forests in the Coromandel coast of India: Vegetation, root biology, mycorrhizae and nutrient cycling. Ph.D dissertation. Pondicherry University, Pondicherry, India.
[39] Parthasarathy, N., and Karthikeyan R. 1997. Plant biodiversity inventory and conservation of two tropical dry evergreen forests on the Coromandel coast, south India. Biodiversity and Conservation 6: 1063-1083.
[40] Venkateswaran, R., and Parthasarathy N. 2003. Tropical dry evergreen forests on the Coromandel coast of India: Structure, composition and human disturbance. Ecotropica 9: 45-58.
[41] Mani, S., and Parthasarathy N. 2005. Biodiversity assessment of trees in five inland tropical dry evergreen forests of peninsular India. Systematics and Biodiversity 3: 1-12.
[42] Ramanujam, M. P., and Kadamban D. 2001. Plant biodiversity of two tropical dry evergreen forests in the Pondicherry region of south India and the role of belief systems in their conservation. Biodiversity and Conservation 10: 1203-1217.
[43] Pallithanam, J. M. 2001. A pocket flora of the Sirumalai Hills, South India. revised and edited by K.M. Matthew. Rapinat Herbarium, St. Joseph's College, Tiruchirapalli, India.
[44] Jayakumar, S., Arockiasamy D. I., and Britto J. S. 2002 Conserving forests in the Eastern Ghats through remote sensing and GIS- A case study in Kolli hills. Current Science 82: 1259-1267.
[45] Balaguru, B., Britto J. S., Nagamurugan N., Natarajan D., Soosairaj S., Ravipaul S., and Arockiasamy D. I. 2003. Vegetation mapping and slope characteristics in Shervarayan Hills, Eastern Ghats using remote sensing and GIS. Current Science 85: 645-653.
[46] Natarajan, D., Britto J. S., Balaguru B., Nagamurugan N., Soosairaj S., and Arockiasamy D. I. 2004. Identification of conservation priority sites using remote sensing and GIS — A case study from Chitteri hills, Eastern Ghats, Tamil Nadu. Current Science 86: 1316-1323.
[47] Gamble, J. S., and Fischer C. E. C. 1915-1935. Flora of the Presidency of Madras. Parts I to XI. Secretary of state for India, London.
[48] Matthew, K. M. 1995. An excursion flora of central Tamil Nadu, India, pp. 682. Oxford & IBH Publishing Company. New Delhi.
[49] Reddy, M. S., and Parthasarathy N. 2003. Liana diversity and distribution in four tropical dry evergreen forests on the Coromandel coast of south India. Biodiversity and Conservation 12: 1609-1627.
[50] Reddy, M. S., and Parthasarathy N. 2007. Liana diversity and distribution on host trees in four inland tropical dry evergreen forests of peninsular India. Tropical Ecology 47: 103-116.
[51] Anbarashan, M., and Parthasarathy N. 2008. Comparitive tree community analysis of two old-growth tropical dry evergreen forests of peninsular India. In: P.C. Trivedi (ed.) Frontiers in Plant Sciences (in press).
[52] Hunneyball, G. 2003. The tropical dry evergreen forest of Tamil Nadu: temple groves, evergreenness and spatial variation. (Unpublished report).
[53] Monk, C. D. 1966. An ecological significance of evergreenness. Ecology 47: 504-505.
[54] Pragasan, A. L., and Parthasarathy N. 2005. Litter production in tropical dry evergreen forests of south India in relation to season, plant life-forms and physiognomic groups. Current Science 88: 1255-1263.
[55] Venkateswaran, R. 2005. Short-term tree population changes, growth and phenology of woody species in tropical dry evergreen forests on the Coromandel coast of India. Ph.D dissertation. Pondicherry University, Pondicherry, India.
[56] Mani, S., and Parthasarathy N. 2007. Tree population and above-ground biomass changes in two tropical dry evergreen forests of peninsular India. Tropical Ecology (ms).
[57] Mani, S. and Parthasarathy N. 2007. Above-ground biomass estimation in ten tropical dry evergreen forest sites of peninsular India. Biomass and Bioenergy 31: 284-290.
[58] Selwyn, M. A., and Parthasarathy N. 2006. Reproductive traits and phenology of plants in tropical dry evergreen forest on the Coromandel coast of India. Biodiversity and Conservation 15: 3207-3234.
[59] Burger, W. C. 1974. Flowering periodicity at four altitudinal levels in eastern Ethiopia. Biotropica 6: 38-42.
[60] Opler, P. A., Frankie G. W., and Baker H. G. 1980. Comparative phenological studies of treelet and shrub species in tropical wet and dry forests in the lowlands of Costa Rica. Journal of Ecology 68: 167- 188.
[61] Bhat D. M. 1992. Phenology of tree species of tropical moist forest of Uttara Kanada district, Karnataka, India. Journal of Biosciences 17: 325-352.
[62] Murali, K. S., and Sukumar R. 1994. Reproductive phenology of a tropical dry forest in Mudumalai, southern India. Journal of Ecology 82: 759—767.
[63] Sundarapandian, S. M., Chandrasekeran S., and Swamy P. S. 2005. Phenological behaviour of selected tree species in tropical forests at Kodayar in the Western Ghats, Tamil Nadu, India. Current Science 88: 805-810.
[64] Selwyn, M. A., and Parthasarathy N. 2007. Fruiting phenology in a tropical dry evergreen forest on the Coromandel coast of India in relation to plant life-forms, physiognomic groups, dispersal modes, and climatic constraints. Flora 202: 371-382.
[65] Koptur, S., Haber W. A., Frankie G. W., and Baker H. G.1988. Phenological studies of shrub and treelet species in tropical cloud forests of Costa Rica. Journal of Tropical Ecology 4: 347—359.
[66] Leiberman, D. 1982. Seasonality and phenology in a dry tropical forest in Ghana. Journal of Ecology 70: 791—806.
[67] Reich, P. B. 1995. Phenology of tropical forests — patterns, causes, and consequences. Canadian Journal of Botany 73: 164—174.
[68] Borchert, R. 1996. Phenology and flowering periodicity of neotropical dry forest species: evidence from herbarium collections. Journal of Tropical Ecology 12: 65—80.
[69] Muchado, I. C. S., Barros L. M., and Sampaio E. V. S. 1997. Phenology of caatinga species at Talhada, PE, northeastern Brazil. Biotropica 29: 57—68.
[70] Swamynathan, B., and Parthasarathy N. 2005. Community-level fruit production and dispersal modes in two tropical dry evergreen forests of peninsular India. Tropical Biodiversity 8: 159-171.
[71] Chandran, M. D. S., and Hughes J. D. 1997. The sacred groves of south India: ecology, traditional communities and religious change. Social Compass 44: 413-427.
|







