|
Research Article
Dung Beetles and Long-term Habitat Fragmentation in Alter do Chão, Amazônia, Brazil
Kevina Vulinec1 * Albertina Pimentel Lima2, Elildo A. R. Carvalho-Jr.2 and David J. Mellow3
1Department of Agriculture and Natural Resources, 1200 N. Dupont Highway, Delaware State University, Dover, Delaware 19901, USA. 2Instituto Nacional de Pesquisas da Amazônia, Coordenação de Pesquisas em Ecologia, Caixa Postal: 478, Aleixo, 69011- 970, Manaus, Amazonas, Brasil. 338 Meadow Ave., Wyoming, Delaware 19934, USA
*Corresponding author email: [email protected]
Abstract
We examined the abundance and diversity of dung beetles in forest fragments within a savanna landscape near Alter do Chão, Pará, Brazil. These fragments have existed for 150 years and possibly millennia. Using pit-fall traps to capture dung beetles, we investigated fragment area, fragment isolation, and tree density in fragments as predictors of species richness, abundance, and biomass of dung beetles. Across six fragments, isolation distance was negatively related with dung beetle species richness, while all other variables were unrelated. We also examined the abundance of the dominant species using flight-intercept traps in 21 fragments. Tree density correlated negatively with abundance of the dominant species, a probable new species.
Key words: Dung beetles; fragmentation; Alter do Chão; Brazil; species-area, habitat isolation.
Resumo
Nós investigamos a abundancia e diversidade dos besouros rola-bosta em fragmentos de floresta próximo a Alter do Chão, Pará, Brasil. Estes fragmentos existem de 150 anos e possivelmente por milênios. Utilizando armadilhas de queda para capturar besouros de esterco, investigamos a área, o isolamento, e a densidade de árvore de besouros de esterco. O isolamento do fragmento foi a única variável independente que significativamente afetou a riqueza de espécie; a abundância, a biomassa, a diversidade, e as medidas de equidade não foram afetadas pelo tamanho de fragmento, isolamento, ou densidade de árvore. Densidade de árvore, contudo, correlacionado negativamente com abundância da espécie dominante, uma provável nova espécie de besouro
Palavras-chave: Besouros de esterco, fragmentação; Alter do Chão; Brasil; espécie-área, isolação de hábitat.
|
Received: 16 January 2008, Accepted : 8 May 2008, Published: 9 June, 2008
Copyright: © 2008 Vulinec et al. This is an open access paper. We use the Creative Commons Attribution 3.0 license http://creativecommons.org/licenses/by/3.0/us/ .The license permits any user to download, print out, extract, archive, and distribute the article, so long as appropriate credit is given to the authors and source of the work. The license ensures that the published article will be as widely available as possible and that your article can be included in any scientific archive. Open Access authors retain the copyrights of their papers. Open access is a property of individual works, not necessarily journals or publishers.
Cite this paper as: Vulinec, K., Pimentel, L.A., Carvalho, E. A. R. and Mellow, D. J.. 2008. Dung Beetles and Long-term Habitat Fragmentation in Alter do Chão, Amazônia, Brazil. Tropical Conservation Science Vol.1 (2):111-121. Available online: tropicalconservationscience.org
Introduction
Habitat fragmentation is considered detrimental to most tropical species. The majority of studies describing the effects of fragmentation on tropical biota have come from projects of recent anthropogenic fragmentation and these studies have shown generally negative effects (e.g., BDFFP in Brazil, Lago Guri in Venezuela; [1-3]). Dung beetles are important in ecosystem functioning and are relatively well-known taxonomically. For these reasons their use as indicator species for habitat disturbance research has increased in recent years (see [6] for a review). Numerous studies have examined how habitat fragmentation and disturbance affect these insects' abundance and diversity. In general, these studies have demonstrated a negative relationship for both abundance and species diversity with increased disturbance or fragmentation [1, 6].
In this study, we surveyed dung beetles in dry forest fragments surrounding the village of Alter do Chão, Pará, Brazil to examine the effects of long-term fragmentation on species diversity and abundance of these beetles. The origin of these fragments is not known; they have persisted longer than other fragments in the Amazon (they are mentioned by Bates [4]), possibly for millennia [5], and thus, they represent a unique opportunity to examine diversity in long-standing areas of fragmentation. We addressed two main questions in our study: (1) Does area, isolation, or the density of trees of a fragment affect species richness, abundance, and biomass, and (2) does fragmentation, isolation, or tree density affect the abundance of the dominant species? Although sampling effort and number of fragments limited the conclusions from this study, it provides preliminary indications that in areas of long-term fragmentation in the Amazon, dung beetle species richness and abundance may be more affected by isolation than fragment size, and vegetation structure may contribute to changes in species community structure. In addition, we were interested in the most dominant species (as yet not identified) because its relative abundance in the dung beetle community at this locality is unusual compared to other Amazon collections [1, 7, 8].
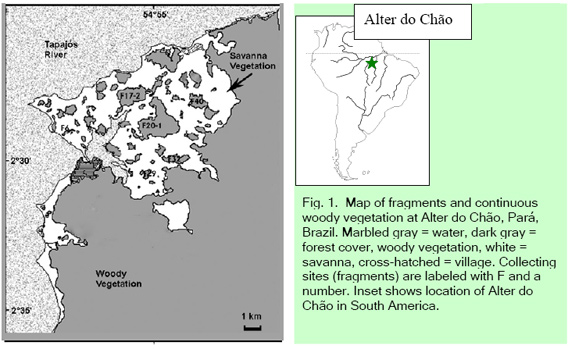
Methods
Study Area
We conducted the study in a 30,000 ha area near the village of Alter do Chão, Pará, Brazil (Lat. 2o31'S, Long. 55o00'W; Fig. 1). Mean annual precipitation in the area is ca. 2,000 mm with a pronounced dry season from June to November [9]. There are many vegetation types in the region, including Amazonian savanna (Fig. 2) [10, 11], forest fragments dispersed in a savanna matrix (Fig. 3), and a continuous mosaic of mature and immature forested vegetation (Unidade De Conservação Chamda Flona, Floresta Nacional Da Amazonia) [12, 13]. Henry Bates visited the region in the middle of the nineteenth century and described the occurrence of forest islands isolated by savanna [4]. These reports indicate that the landscape has been fragmented for at least 150 years and probably much longer. The origin of the savanna and forest fragments is not understood, but may have been caused by Amerindian fires [12] or climatic changes over several thousand years [14]. Sanaiotti et al. [5] suggest that the region had continuous cover of arboreal vegetation about 2,000 years ago.

The savanna in this area is dominated by the grasses Paspalum carinatum Flugge and Trachypogon plumosus Nees, and large clumps of shrubs and trees, mostly of species from the Myrtaceae and Rubiaceae. [12]. The forest fragments are dominated by trees of the families Myrtaceae, Flacoutiaceae, and Leguminosae (W.E. Magnusson and J. Camara, unpubl. data). Large mammals seen fairly frequently in the fragments include: Red and gray brocket deer (Mazama americana and M. gouazoubira), collared peccary (Pecari tajacu), dusky titi monkey (Callicebus moloch), silvery marmoset (Mico argentata), red-handed howler monkey (Aloutta discolor), paca (Cuniculus paca), agouti (Dasyprocta leporina), and nine-banded armadillo (Dasypus novencinctus) [15]. This area is unique in the Amazon Basin and has been suggested for protection as a faunal reserve [16].

Landscape Metrics
We determined fragment size and distance to nearest large forest area from satellite photography, digitized using the CAMRIS program [17]. Digitizing was based on a Landsat TM5 image, previously georeferenced with IDRISI 32 software [18]. Large forests were defined as the continuous forest or any fragment larger than 100 ha, assumed to be similar to continuous forest from a dung beetle's perspective [19]. We used edge-to-edge distance to nearest large forest area as a metric for isolation [20].
Sampling of Dung Beetles
We conducted fieldwork during 2004 at the end of the wet season (28 May-12 June). We set baited pitfall traps in a subset of six fragments ranging in size from 8.5 to 360.4 ha (Table 1). During this collection, we set 10 traps per fragment, separated by 50 m [21], over three days at each of six fragments. Fragments were numbered using an existing system [12]. Logistics prevented sampling of the continuous forest at this time. These traps consisted of 1 liter plastic cups buried flush with the soil. We suspended a five ml cup with human dung above the traps [7, 21]. Beetles were captured alive for behavioral observations (not reported here); a plastic funnel with a 5 cm aperture kept them from escaping the trap. We employed the same collecting effort in all six fragments. Biomass of each species, except for Dichotomius sp. 1, was from Vulinec [22]; this one species was weighed at the Instituto Nacional de Pesquisas da Amazônia in Manaus (APL and EARC) (Table 2). We calculated the dependent variables: species richness (number of species), abundance (number of individuals of all species), and mean biomass (g/trap = (S individual mass X abundance) per trap) [23].
Table 1. Fragment name, size, number of species, total number of individuals, distance to nearest large fragment or continuous forest> 100 ha, density of trees (number/m2), species richness (from collection), Estimated species richness from Chao 1).
|
Fragment
|
Area (ha)
|
Distance to nearest large forest (km)
|
Density of trees (#/ha)
|
# Species
|
# Individuals
|
Total Biomass (g)
|
Estimated Species Richness (Sest)
|
|
F20-1
|
360.4
|
0.3
|
214
|
14
|
986
|
61.37
|
14
|
|
F17-2
|
189.8
|
0.3
|
871
|
15
|
1836
|
124.34
|
15
|
|
F40
|
66.4
|
1
|
111
|
10
|
824
|
56.36
|
10
|
|
F32
|
59.7
|
0.1
|
151
|
13
|
1182
|
68.38
|
13
|
|
F29
|
14.1
|
0.6
|
165
|
12
|
902
|
53.29
|
15
|
|
F6
|
8.5
|
2.8
|
1020
|
8
|
144
|
6.09
|
8
|
|
|
|
|
|
|
|
|
|
We set flight-intercept traps during the 2001 dry season (from August to December) to determine the abundance of the dominant species, Dichotomius sp. 1 in the fragments. This species was the only dung beetle caught in this type of trap, is the most common species in the fragments, and has not been collected in the savanna (Lima, unpublished data). For this analysis, we sampled 21 forest patches, ranging in size from 3.6 to 360 ha. We established four 250 m long parallel transects, separated by 50 m and placed along the longest axis of fragments. We placed five flight-intercept traps on each transect (250 m), at 50 m intervals (20 traps per site) to collect beetles. These traps were made from a 23 cm x 25 cm transparent plastic screen originating at ground level which we placed vertically over a buried two-liter plastic bottle. A 20 cm x 10 cm opening was cut along one side of the bottle, which was buried on its side in the soil so that the long axis of the opening was parallel to, and flush with, the soil. Each bottle was filled with 100 ml of a 1% formalin solution and three drops of detergent per liter. We deployed the traps for 48 hours. Due to logistic considerations, traps had to be set on different days.
Sampling of vegetation
We counted the number of trees in four 250 m x 2 m plots (total area = 0.2 ha) in each of the 6 fragments. These data are reported as tree density (#trees/ha).
Data Analysis
We used Pearson Product Moment Correlation to examine if there were any correlations among the three independent variables, fragment area, isolation, or density of trees. We examined the association between the dependent variables (species richness, abundance, mean biomass, and three independent variables: size of fragment (log-transformed), isolation of fragment, and density of trees in a fragment using multiple linear regressions [27]. To determine the completeness of sampling, we plotted Coleman rarefaction curves and number of observed species for each fragment using EstimateS Version 7.5 [24] and calculated the estimated total number of species using Chao 1 [25]. We used a sample-based assessment protocol with rescaling of the x-axis to number of individuals [26]. We compared the abundance from flight-intercept traps of the most common species in the fragments (Dichotomius sp. 1) and examined fragment area (log-transformed), density of trees, and isolation with abundance of this beetle with Spearman Rank Order Correlation. We used SigmaStat 3.0 for statistical tests.
Table 2. Dung beetle species collected, number of individuals at each fragment, total number of individuals, biomass of one individual, and total biomass of each species caught. Biomass from [7, 29]. Area of fragments: F20-1 (360.4 ha), F17-2 (189.8 ha), F40 (66.4 ha), F32 (59.7 ha), F29 (14.1 ha), F6 (8.5 ha)
.
Results
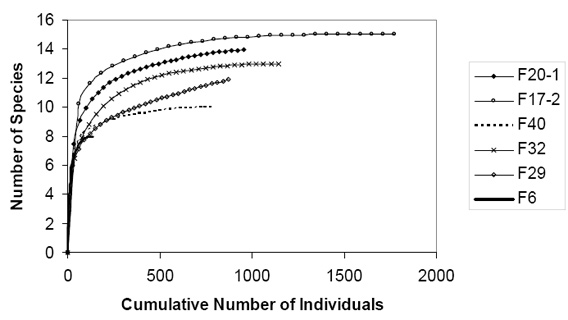
With baited pitfall traps, we caught 17 total species in the six fragments (Mean = 12; SD = 2.61; Table 2) and 5874 total individuals (Mean = 979; SD = 548.24; Table 2). Most of our rarefaction curves of species accumulation over number of samples reached a relative asymptote between 500 and 1,000 individuals, indicating that we collected nearly all species in all fragments except F29 (14.1 ha), which did not reach an asymptote, and F6 (8.5 ha) which did, but at around 100 individuals (Fig. 4).
The independent variables were not correlated with each other (area vs. isolation: R = -0.695, P = 0.125; area vs. tree density: R = -0.231, P = 0.66; tree density vs. isolation: R = 0.609, P = 0.199). The multiple regressions showed that beetle species richness was not related to log transformed area of fragment or tree density, but was significantly related to isolation (area: t = 0.686, P = 0.563; tree density: t = 2.76, P = 0.11; isolation: t = -4.568, P = 0.045). Abundance was not related to any of the three variables, although isolation was nearly positively significant (Area: t = -0.054, P = 0.961; tree density: t = 2.736, P = 0.112; isolation: t = -3.802, P = 0.063). Biomass was not related to any variable (Area: t = 0.218, P = 0.847; tree density: t = 2.084, P = 0.173; isolation: t = -2.611, P = 0.121).
Density of trees in a fragment may indicate openness of the habitat and have an influence on species occurrence. There was a negative and significant relationship between the density of trees and the number of Dichotomius sp. 1 caught in the flight-intercept traps (Spearman Rank Order Correlation Coefficient = -0.55, N = 22, P = 0.008).
Discussion
Species in fragments are dependent on nearby areas for colonists. Volant animals, such as bats, appear to move readily between the fragments of Alter do Chão [28]. Generally, little is known about dung beetle dispersal over distances, although recent evidence indicates that the dung beetle fauna recovers from disturbance relatively quickly, indicating the ability to disperse readily [19]. Nevertheless, beetles in anthropogenic islands in Lago Guri, Venezuela, disperse from islands but do not recolonize them, leaving these sites depauperate in dung beetles [29]. The habitat patches of Alter do Chão are not far apart and many are within 0.5 km of the continuous forest or large fragments; it seems reasonable to assume that beetles can fly among them. They are also not surrounded by water as is the case in Lago Guri. We do not know if fragment communities at Alter do Chão are limited by lack of colonizers; however, the most isolated patches showed a significant decrease in species richness.
The dominant beetle in the forest fragments around Alter do Chão is Dichotomius sp. 1, an as yet unidentified species related to the D. lucasi group (F. Genier, pers. comm.). Dichotomius sp. 1 may be more tolerant of disturbed conditions than other dung beetles. Abundance of this beetle was significantly negatively correlated with tree density. This species does not occur in savanna (Lima, unpublished data). Vulinec [7] demonstrated that Amazonian Dichotomius species in general are more abundant in slightly disturbed secondary forest than in primary intact forest, up to a point of moderate disturbance where their numbers rapidly decline. This abundance pattern suggests that this group has a greater tolerance of some disturbed conditions. Possibly, this habitat tolerance is the same for our Alter do Chão species.
Our collected species richness paralleled estimated species richness calculated as Chao 1 [29] (Table 1), indicating that we had probably collected most of the species in all fragments except F29. In our system, isolation of fragments appeared to be the most important variable explaining dung beetle species richness. Different dispersal abilities among species would limit those that could colonize the furthest fragments. Other studies have shown conflicting results when isolation and dung beetle species richness are compared. Chapman et al. [30] found no effect of isolation on richness, but the opposite was found by Larsen et al. [29]. The abundance of the one dominant species at Alter do Chão was very significantly related to tree density; Estrada et al. [31] also found dung beetle species abundance correlated with complexity in vegetation. In general, the majority of studies examined in a meta-analysis have found a decrease in species richness and diversity with fragment size [6]; however, we did not find this relationship. The age of the fragments in our study may have an effect on species richness. Other studies have indicated a decrease in species richness with age, but these studies examined fragments that had only been recently created [29, 30]. In addition, fragments with a surrounding matrix that has some cover or secondary growth appear to support higher diversity than fragments in an inhospitable matrix [28, 30, 32]. The fragments at Alter do Chão are surrounded by natural savanna with numerous small trees and shrubs, which may mediate strong species-area effects [19]. Numerous large mammals, which provide a source of food for dung beetles, have also been documented from within these fragments and nearby forest tracts [15].
Implications for Conservation
Our findings underscore the importance of isolation in fragmentation studies [6]. Species richness declined with increasing fragment isolation. We did not observe any extreme difference in abundance of any species with fragment size or isolation. Most dung beetle studies reviewed in Nichols et al. [6] show a relationship between species richness and fragment size and isolation. In addition, habitat variables, such as tree density, may be important predictors of species occurrences. Because pitfall traps were not set in the continuous forest or in the savanna matrix, this study is limited in its scope. Nevertheless, unpublished data (Lima) suggest that, similar to other areas in the Amazon Basin, there is little overlap between the species collected in savanna and forest habitat [7]. In addition, certain species that are quite common in other Amazon sites, such as Canthon aequinoctialis and Onthophagus bidentata (Fig. 5), are relatively rare in these fragments [22]. Total number of species and total abundance of beetles were also much lower than in most Amazonian rainforest sites [7, 33]. In Mexico, Halffter et al. [34] found that areas with fragmentation of at least 700 years had gained back some species richness over areas of recent fragmentation. Nevertheless, the species that occurred in these fragments tended to be diurnal species and those that are more tolerant of human disturbance. Whether results from Alter do Chão can be generalized to other sites with long-standing fragments will require similar studies in other locales.
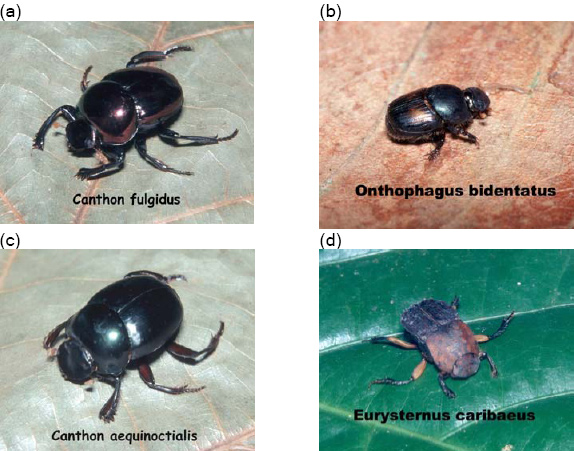
Fig. 5. (a) Canthon fulgidus Redtenbacher 1867, one of the common beetles in fragments at Alter do Chão; (b) Onthophagus bidentatus, Drapiez 1819, an uncommon species in fragments; (c) Canthon aequinoctialis Harold 1868, an uncommon species in fragments; (d) Euysternus caribaeus Herbst 1789, a common species in fragments (Photos courtesy of David Jordan).
This area has a long history of family agriculture. The local inhabitants come from different regions of Brazil, and 30% are descendents of indigenous peoples (Lima, unpub.) (Fig. 6). Currently, the village near the fragments and savanna matrix is used extensively for tourism. There is extraction from the fragments and some hunting pressure. Nevertheless, several species of large mammals (e.g., howler monkeys, dusky titi monkeys, gray brocket deer, collared peccary) persist in these fragments (Sampaio, Lima, and Magnusson, in preparation), which also may allow dung beetles to subsist. The multiple use of the habitat around the village may be supported in the future with certain restrictions. At least for dung beetles, this area has a unique faunal structure and is of major interest as a site of long-term fragmentation and human habitation.

Fig. 6. (a) An example of the unique crafts from the Tapajós region; (b) Ediwaldo Vasconcellos and family, descendents of the original peoples of the Alter do Chão region. (Photos courtesy of Betty Ferster).
We suggest our study provides a basis for further investigation of the mechanisms that influence community structure and particularly, what characteristics allow a species to persist in a fragmented habitat. We suggest that certain characteristics, such as dispersal ability or tolerance to low humidity, may allow some species to respond positively to long-term fragmentation. We also suggest that studies of fragmentation examine individual species reactions to habitat disturbance and isolation in addition to overall species richness and abundance. Given the importance of dung beetles in ecosystems as waste recyclers, seed dispersers, and parasite controls [1, 29, 30, 35], conservation of this group is essential in rapidly shrinking habitat, and ultimately depends on the conservation of large mammals.
Acknowledgments
We thank the following organizations for their support: Women's International Science Collaboration, (AAAS & NSF) and Delaware State University Professional Development Fund. In Brazil, we are grateful to O Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Instituto Nacional de Pesquisas da Amazônia (INPA). We thank Ana Albernaz and residents of Alter do Chão who made logistics of this study feasible. We are especially grateful to Sam Kahn for his valuable assistance. We also thank François Genier for help with identifications and comments on the manuscript and three anonymous reviewers for invaluable suggestions on an earlier draft. This project would not have been accomplished without the continual aid and friendship of our Brazilian field assistant, Ediwaldo Vasconcelos (Edi) and his family.
References
[1] Klein, B. C. 1989. Effects of forest fragmentation on dung and carrion beetle communities in Central Amazonia. Ecology 70: 1715-1725.
[2] Laurance, W. F., Ferreira, E. V., Rankin-de Merona, J. M., and Laurance, S. G. 1998. Rain forest fragmentation and the dynamics of Amazonian tree communities. Ecology 79: 2032-2040.
[3] Terborgh, J., Lopez, L., Nuñez, P., Rao, M., Shahabuddin, G., Orihuela, G., Riveros, M., Ascanio, R., Adler, G. H., Lambert, T. D., Balbas, L. 2001. Reports Ecological Meltdown in Predator-Free Forest Fragments. Science 294: 1923 — 1926.
[4] Bates, H. W. 1863. The Naturalist on the River Amazons. John Murray, London, England.
[5] Sanaiotti, T. M., Martinelli, L. A., Victoria, R. L., Trumbore, S. E., and Camargo, P. B. 2002. Past vegetation changes in Amazon savannas determined using carbon isotopes of soil organic matter. Biotropica 34: 2-16.
[6] Nichols, E., Larsen, T., Spector, S., Davis, A., Escobar, F., Favila, M., Vulinec, K. 2007. Dung beetle response to tropical forest modification and fragmentation: a quantitative review and meta-analysis. Biological Conservation 137: 1-19.
[7] Vulinec, K. 2002. Dung beetle communities and seed dispersal in primary forest and disturbed land in Amazonia. Biotropica 34: 297-309.
[8] Scheffler, P. Y. 2005. Dung beetle (Coleoptera: Scarabaeidae) diversity and community structure across three disturbance regimes in eastern Amazonia. Journal of Tropical Ecology 21: 9-19.
[9] Miranda, I. S. 1993. Estructura do estrato arbóreo do cerrado amazônica em Alter do Chão, Pará, Brasil. Revista Brasileira de Botânica 16: 143-150.
[10] Huber, O. 1982. Significance of savanna vegetation in the Amazon Territory of Venezuela. In G. T. Prance (Ed.). Biological Diversification in the Tropics, pp. 221-244. Columbia University Press, New York, New York, USA.
[11] Pires, J. M., and Prance, G. T. 1985. The vegetation types of the Brazilian Amazon. In G.T. Prance and T.E. Lovejoy (Eds.) Key Environments: Amazonia, pp. 109-145. Pergamon Press, Oxford, England.
[12] Sanaiotti, T. M., and Magnusson, W. E. 1995. Effects of annual fires on the production of fleshy fruits eaten by birds in a Brazilian Amazonian savanna. Journal of Tropical Ecology 11: 53-65.
[13] Magnusson, E. W., Lima, A. P., Faria, A. S., Victoria, R. L., and Matinelli, L. A. 2001. Size and carbon acquisition in lizards from Amazonian savanna: evidence from isotope analysis. Ecology 82: 1772-1780.
[14] Freitas, H. A., Pessenda, L. C. R., Aravena, R., Gouveia, S. E. M., Ribeiro, A. S., and Boulet, R. 2001. Late Quaternary vegetation dynamics in the southern Amazon basin inferred from carbon isotopes in soil organic matter. Quaternery Research 55: 39-46.
[15] Sampaio, R. 2007. Efeitos a longo prazo da perda do habitat e da caça sobre mamíferos de médio e grande porte na Amazônia Central. Dissertation, Universidade Federal do Amazonas e Instituto Nacional de Pesquisas da Amazônia.
[16] Rylands, A. B. and Pinto, L. P. S. 1998. Conservação da biodiversidade na Amazônia brasileira: Uma análise do sistema de unidades de conservação. Cadernos, Fundação Brasileira para o Desenvolvimento Sustentável-FBDS, No.1, Rio de Janeiro, Brasil.
[17] Ford, R.G. 1993. CAMRIS- Computer Aided Mapping and Resource Inventory System, Unpublished.
[18] Eastmann, J. R. 1995. IDRISI for Windows. Clark Labs for Cartographic Technology and Geographic Analysis. Worcester, Massachusetts, USA.
[19] Quintero, I. and Roslin, T. 2005. Rapid recovery of dung beetle communities following habitat fragmentation in central Amazonia. Ecology 86: 3303-3311.
[20] McEuen, A. B. and Curran, L. M. 2006. Plant recruitment bottlenecks in temperate forest fragments: seed limitation and insect herbivory. Plant Ecology 184: 297-309.
[21] Larsen, T., and Forsyth, A. 2005. Trap spacing and transect design for dung beetle biodiversity studies. Biotropica 37: 322-325.
[22] Vulinec, K. 1999. Dung Beetles (Coleoptera: Scarabaeidae), Monkeys, and Seed Dispersal in the Brazilian Amazon. PhD dissertation. University of Florida, Gainesville, Florida, USA.
[23] Gardner. T. A., Hernández, M. I. M., Barlow, J., and Peres, C. A. 2008. Understanding the biodiversity consequences of habitat change: the value of secondary and plantation forests for neotropical dung beetles. Journal of Applied Ecology doi: 10.1111/j.1365-2664.2008.01454.x.
[24] Colwell, R. K. 2005. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 7.5. User's Guide and application published at: http://purl.oclc.org/estimates.
[25] Chazdon, R. I., Colwell, R. K., Denslow, J. S., and Guariguata, M. R. 1998. Statistical methods for estimating species richness of woody regeneration in primary and secondary rain forests of NE Costa Rica. In F. Dallmeier, and J. A. Cominsky (Eds). Forest Biodiversity Research, Monitoring and Modeling Conceptual Background and Old World Case Studies, pp 285—309. Parthenon Publishing, Paris, France.
[26] Magurran, A. 2006. Measuring biological diversity. Blackwell Publishing, Malden, Massachusetts, USA.
[27] Gotelli, N. J. and Ellison, A. M. 2004. A Primer of Ecological Statistics. Sinauer Associates Inc., Sunderland Massachusetts.
[28] Bernard, E. and Fenton, M. B.. 2007. Bats in a fragmented landscape: Species composition, diversity and habitat interactions in savannas of Santarém, Central Amazonia, Brazil. Biological Conservation 134: 332-343.
[29] Larsen, T., Williams, N. M., and Kremen, C. 2005. Extinction order and altered community structure rapidly disrupt ecosystem functioning. Ecology Letters 8: 538-547.
[30] Chapman, C. A., Chapman, L. J., Vulinec, K., Zanne, A., and Lawes, M. J. 2003. Fragmentation and alteration to seed dispersal processes: dung beetles, seed fate, and seedling diversity. Biotropica 35: 382-393.
[31] Estrada, A., Coates-Estrada, R., Anzures, A. and Cammarano, P. 1998. Dung and carrion beetles in tropical rain forest fragments and agricultural habitats at Los Tuxtlas, Mexico. Journal of Tropical Ecology 14: 577-598.
[32] Feer, F. and Hingrat ,Y. 2005. Effects of forest fragmentation on a dung beetle community in French Guiana. Conservation Biology 19: 1103-1112.
[33] Vulinec, K., Lambert, J. E. and Mellow, D. J. 2006. Primate and dung beetle communities in secondary growth rainforests: Implications for conservation of seed dispersal systems. International Journal of Primatology 27: 855-879.
[34] Halffter, G. Favila, M. E., and Halffter V. 1992. A comparative study of the structure of the scarab guild in Mexican tropical rain forests and derived ecosystems. Folia Entomologica Mexicana 84: 131-156.
[35] Losey, J. E. and M. Vaughan. 2006. The economic value of ecological services provided by insects. Bioscience 56:311-323.
|








